Figure 4.
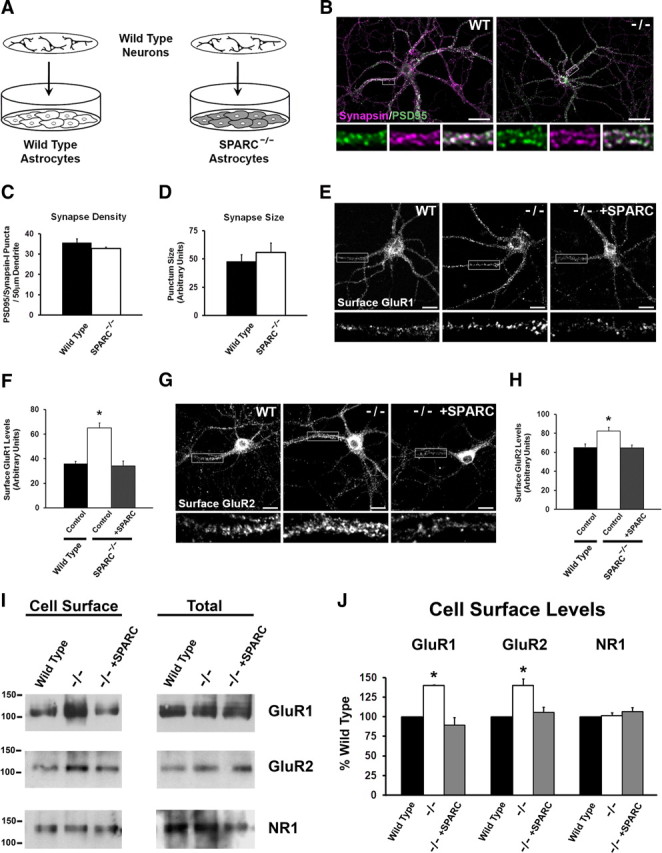
Neurons grown with SPARC KO astrocytes develop normally but have increased GluR1 and GluR2 surface expression. A, Diagram illustrating the culturing of WT neurons above a feeder layer of WT or KO astrocytes. B, Immunostaining showing the colocalization of presynaptic synapsin (magenta) and postsynaptic PSD-95 (green) to reveal synaptic puncta in WT and SPARC-deficient cultures. C, D, Quantification of synaptic punctum density and size. There were no detectable differences between WT and KO cultures [2-tailed t test, p = 0.0912 for density, p = 0.1563 for size (n = 4)]. E, F, Representative images of surface GluR1 expression showing increased intensity of puncta in SPARC-deficient cultures compared to WT cultures. The boxed region is magnified below each image. Recombinant SPARC application (+SPARC; 0.5 μg/ml, 48 h) rescues surface GluR1 levels in SPARC-deficient cultures [ANOVA with post hoc Holm–Sidak test, *p = 0.00025 (n = 3)]. G, H, Representative images of surface GluR2 expression showing increased intensity of puncta in SPARC-deficient cultures compared to WT cultures. The boxed region is magnified below each image. Application of recombinant SPARC (0.5 μg/ml, 48 h) restores surface GluR2 levels to WT levels [ANOVA with post hoc Holm–Sidak test, *p = 0.00246 (n = 4)]. I, J, Representative immunoblots showing cell surface (left panel) and total GluR1, GluR2, and NR1 (right panel) levels in WT, SPARC-deficient, and SPARC-deficient cultures treated with SPARC (0.5 μg/ml, 48 h). Cell surface receptor levels were determined by cell surface biotinylation. Neurons cultured with SPARC KO astrocytes had significantly higher surface GluR1 [ANOVA with post hoc Holm–Sidak test (n = 3), *p = 0.003] and GluR2 (*p = 0.008) but not NR1 (p = 0.472) levels without an increase in total receptor levels. Abnormal levels of GluR1 and GluR2 in the SPARC KO condition could be completely rescued with recombinant SPARC. Scale bars: B, 40 μm; E, G, 20 μm.
