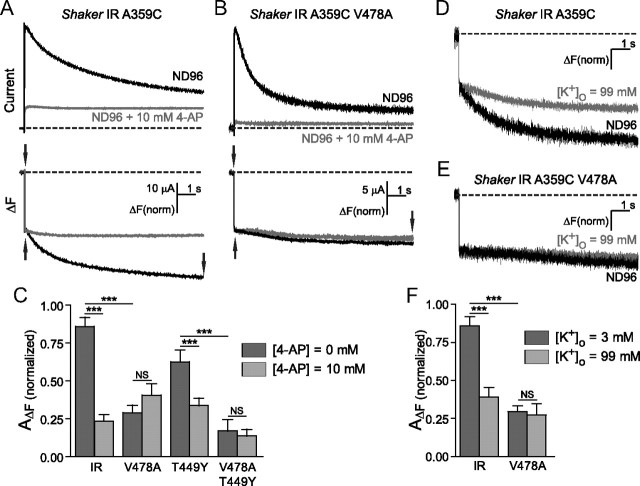
Figure 4.
V478A mutants and 4-aminopyridine similarly prevent C-type inactivation by destabilization of the channel open state. A, B, Currents (top) and fluorescence from Shaker IR A359C (A) and Shaker IR A359C V478A (B), in ND96 (black) and in ND96 + 10 mm 4-aminopyridine (gray), in response to pulses from +60 mV from −80 mV. Slow fluorescence deflection amplitudes are normalized to the amplitudes of the rapid fluorescence components of each signal (amplitudes were measured between points denoted by arrows). C, Normalized amplitudes of slow fluorescence signals from Shaker IR A359C and A359C V478A, and Shaker IR A359C T449Y and A359C T449Y V478A. Fluorescence amplitudes of the fast component were normalized to a value of 1, then slow amplitudes were quantified by normalization to the fast components from the same traces, in ND96 (dark gray) and 10 mm 4-AP (light gray). D, Fluorescence traces from Shaker IR A359C in the presence of ND96 (black) and ND96 containing 99 mm (gray). E, Fluorescence traces from Shaker IR A359C V478A in the presence of ND96 (black) and ND96 containing 99 mm external K+ (gray). F, Slow fluorescence amplitudes recorded from channels in the presence of ND96 (dark gray) and ND96 with 99 mm external K+ (light gray) are normalized to fast amplitudes within the same traces, and then plotted against each other for comparison. Comparisons were made using one-way ANOVA with Bonferroni post hoc tests for significance, ***p < 0.001 or p > 0.05 (NS, not significantly different).