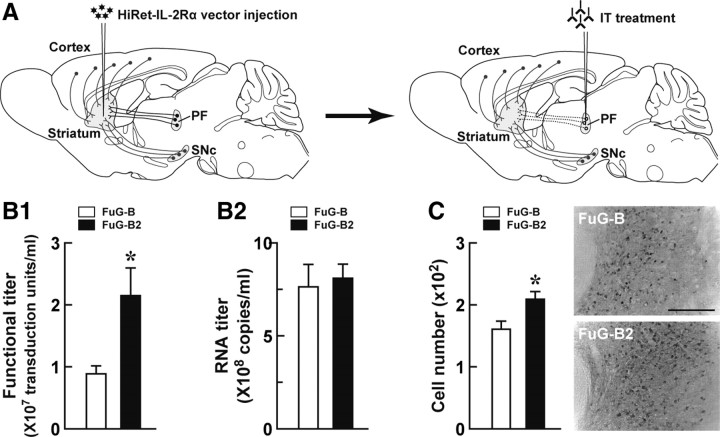
Figure 1.
Strategy for selective neural pathway targeting and improvement of the gene transfer efficiency of the HiRet vector. A, Selective targeting of the thalamostriatal pathway. Injection of the HiRet vector encoding human IL-2Rα into the dorsolateral striatum causes expression of transgene products in various neural pathways innervating the striatum. Subsequent treatment by injection of recombinant IT into the PF of the vector-injected animals induces selective elimination of the neural pathway arising from the PF and innervating the striatum. Dotted lines indicate the removal of the targeted neural pathway. B, Titration of the pseudotyped vectors. HEK293T cells in 18 10-cm tissue culture dishes were transfected with the envelope plasmid encoding FuG-B2 or FuG-B together with the transfer plasmid encoding IL-2Rα–GFP and the packaging plasmids. Vector particles were pelleted by centrifugation and resuspended in 1 ml of PBS. HEK293T cells were transduced with proper concentrations of viral vectors, and the functional titer was determined by flow cytometry (B1). The RNA titer in vector preparations was measured by qRT-PCR analysis (B2). Values were obtained from four independent experiments. *p < 0.05, significant difference from the functional titer of the FuG-B vector (Student's t test). C, In vivo gene transfer of the pseudotyped vectors. Viral vectors with FuG-B or FuG-B2 with equivalent copy numbers of viral RNA (1.2 × 1010 copies/ml, 1.0 μl at four sites) were injected into the dorsolateral striatum of mice (n = 4 for each group). Five weeks later, sections through the PF were used for immunohistochemistry with anti-GFP antibody. The left shows the number of GFP-positive cells per section, and the right indicates representative images of transgene expression in the PF. *p < 0.05, significant difference from the cell number of the FuG-B vector (Student's t test). Scale bar, 200 μm.