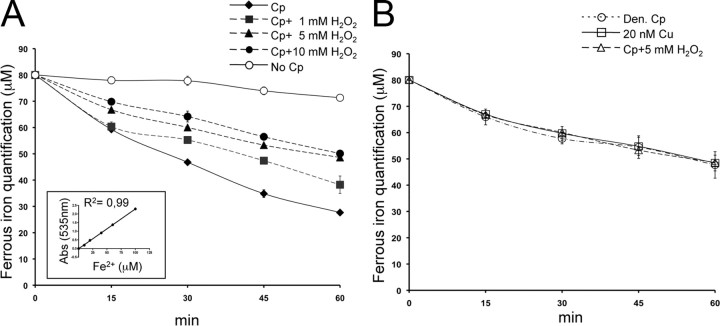
Figure 5.
Oxidation decreases Cp ferroxidase activity. A, Ferroxidase activity was analyzed by Btp assay. Purified Cp (1.25 μg) was incubated with 60 μm FeCl3 (ferrous form) and analyzed at five different times (0, 15, 30, 45, 60 min) with a solution of 1 mm Btp. The decrease in absorbance at 535 nm of Btp-Fe2+ complex is due to ferrous iron oxidation into ferric form (Fe3+). Ferroxidase activity was also analyzed after oxidation by H2O2 treatments (1, 5, 10 mm). By way of control, the assay was performed in the presence of buffer alone (No Cp). Means with SE are indicated (n = 5). The inset shows linear regression of the Btp-Fe2+ complex optical density at 535 nm with different Fe2+ micromolar concentrations. B, Btp assay performed with Cp after 50 mm H2O2 treatments, with heat-denatured Cp (Den Cp), and with buffer alone containing 20 nm Cu2+, the concentration reached by the Cu released from 1.25 μg of denatured Cp.