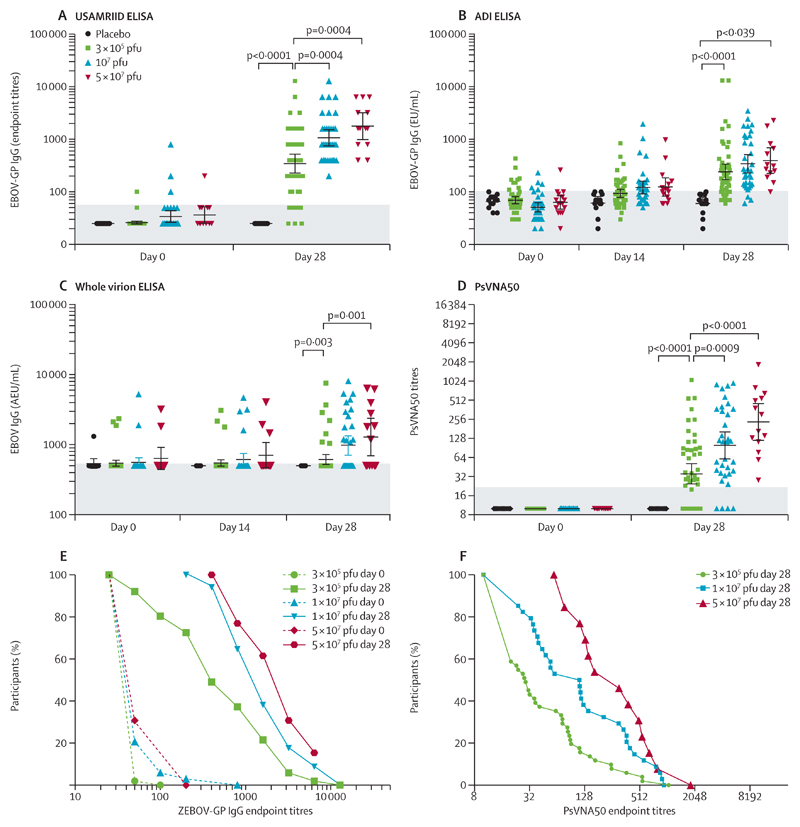
Figure 3. Glycoprotein antibody titres by vaccine dose and assay.
Individual antibody titres assessed at baseline and 28 days after injection by dose group. Results from high-dose vaccinees, previously reported,9 are provided for comparison. Antibodies were measured by ELISA against the homologous glycoprotein of Zaire-Kikwit strain (USAMRIID [A], ADI [B]) or inactivated whole virions of the Zaire-Makona strain (C). Data are geometric mean concentration of endpoint titres (A) or of AEUs per mL (B, C) with 95% CIs. The shaded zones indicate the quantification thresholds. Neutralising antibodies were detected with rVSV pseudovirions complemented by homologous glycoprotein (D). Geometric mean titres and 95% CIs are shown for each dose group and timepoint assessed. The shaded zone indicates the quantification threshold. The results of USAMRIID glycoprotein-ELISA (E) and pseudovirion neutralisation assay (F) were expressed as the reciprocal of the highest dilution showing a positive result. The curves represent the distribution of individual antibody titres in each dose group. Dotted lines indicated baseline titres (E). USAMRIID=US Army Medical Research Institute of Infectious Diseases. ADI=Alpha Diagnostic International. EU=ELISA units. AEU=arbitrary ELISA units. PsVNA50=pseudovirion neutralisation assay. pfu=plaque-forming units. ZEBOV-GP=Zaire Ebola virus glycoprotein.