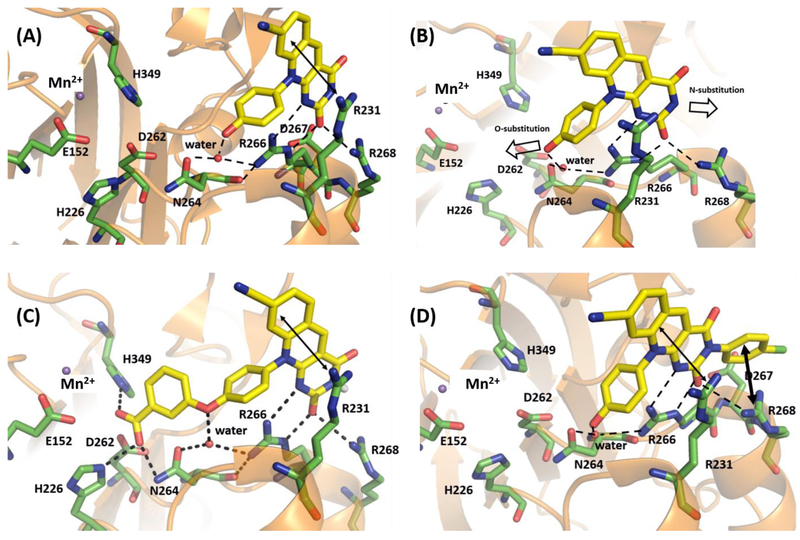
Figure 5.
Molecular modeling of 11k and 12b. (A) Binding mode of 4c within the crystal structure of catalytic domain of humanized mouse TDP2 (PDB code: 5J4218). (B) Potential vectors for designing novel deazaflavin inhibitor types. (C) Predicted binding mode of 11k within the catalytic domain of humanized mouse TDP2. (D) Predicted binding mode of 12b within the catalytic domain of humanized mouse TDP2. Key residues are highlighted in green sticks. H-bond interactions are depicted as black dotted lines. Cation- π and π-π interaction are represented as double headed arrow in black. Water molecule and magnesium ion were represented as red and blue non-bonded sphere. All the residue numberings are based on the human TDP2.