Table 2.
TDP2 inhibitory activity, PAMPA permeability and cytotoxicity for analogues from phenolic OH modifications (11a-m).
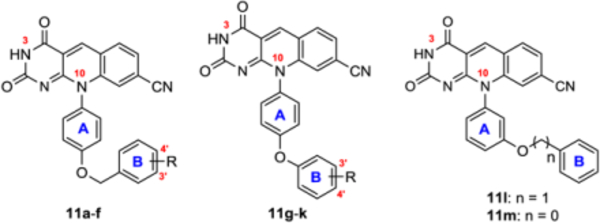 | |||
|---|---|---|---|
| Compd | R | TDP2 IC50 (μM)a | PAMPA Pe (10−6 cm/s)b |
| 4c | -- | 0.14 ± 0.01 | 0.008 |
| 11a | H | 0.20 ± 0.02 | 1.2 |
| 11b | 4′ Cl | 1.6 ± 0.2 | 0.4 |
| 11c | 4′ Br | 1.3 ± 0.0 | 0.2 |
| 11d | 3′ Cl | 0.99 ± 0.09 | 0.4 |
| 11e | 3′ CO2Me | 0.15 ± 0.01 | 0.6 |
| 11f | 3′ CO2H | 0.13 ± 0.01 | 0.01 |
| 11g | H | 1.1 ± 0.1 | 2.8 |
| 11h | 4′ Cl | >10 | 0.8 |
| 11i | 4′ F | 3.8 ± 0.3 | 2.7 |
| 11j | 3′ CO2Me | 0.93 ± 0.05 | 0.7 |
| 11k | 3′ CO2H | 0.19 ± 0.01 | 0.003 |
| 4b | -- | 0.041 ± 0.004 | 0.01 |
| 11l | -- | 0.38 ± 0.02 | 1.7 |
| 11m | -- | 0.30 ± 0.00 | 2.0 |
IC50: concentration of a compound producing 50% inhibition, expressed as mean ± standard deviation from three independent experiments performed in triplicate.
Pe: effective permeability coefficients determined using the PAMPA, measured in five replicates.
