Fig. 6.
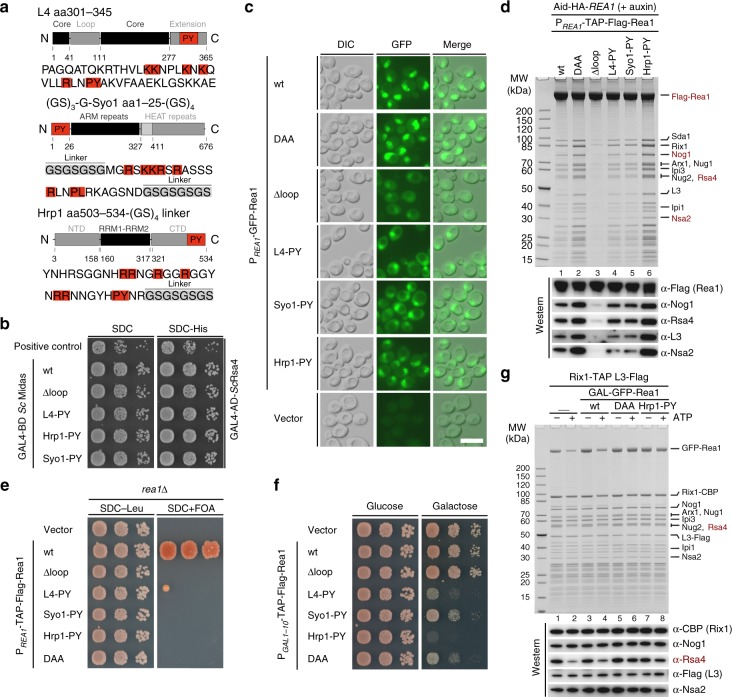
Nuclear import of Rea1 can be restored by a heterologous PY-NLS but still lacks Rea1-MIDAS loop-specific functions. a The PY-NLS containing Rea1-MIDAS element II loop (residues 4657–4696) was exchanged with PY-NLS-containing regions derived from either L4, Syo1, or Hrp1, whose domain organizations are shown. The different PY-NLS-containing fragments used to insert into the Rea1-MIDAS Δloop construct are highlighted in red and their amino acid sequences are shown below. Additional linker sequences are labeled and marked in gray. b Yeast two-hybrid analysis of the interactions between Rsa4 and the indicated Rea1-MIDAS constructs from S. cerevisiae. c Subcellular localization of the indicated N-terminally GFP-tagged Rea1 constructs under control of the endogenous REA1 promotor was monitored by fluorescence microscopy. Nomarski (DIC), GFP and merged images are shown. Scale bar: 5 µm. d Affinity purification of the Rea1-MIDAS Δloop constructs containing foreign PY-NLSs from L4, Syo1, or Hrp1. The indicated Rea1 constructs were fused to an N-terminal TAP-Flag tag under the endogenous REA1 promotor, which were transformed into an AID-HA-REA1 degron strain. Rea1 depletion was induced by addition of auxin (500 µM final concentration) for 2 h before harvesting and subsequent affinity purification. Final eluates were analyzed by SDS-PAGE following Coomassie staining or by western blot analysis using the indicated antibodies. e The indicated Rea1 constructs were transformed in a rea1∆ shuffle strain and the viability of the respective mutants assessed on 5-FOA-containing plates. f The indicated Rea1 constructs under control of the GAL1-10 promoter or an empty vector control were transformed in a wild-type strain (expressing endogenous REA1) and cell growth was monitored on plates containing glucose (SDC-Leu, left) or galactose (SGC-Leu, right). g Rix1-TAP pre-60S particles isolated after overexpression of the indicated Rea1 constructs under control of the GAL1-10 promotor (lanes 3–8) were incubated with 2.5 mM ATP (lanes 2, 4, 6, 8) or left untreated (lanes 1, 3, 5, 7) before re-isolation via L3-Flag. Final Flag-eluates were analyzed by Coomassie staining or by western blotting using the indicated antibodies. Source data are provided as a Source Data file