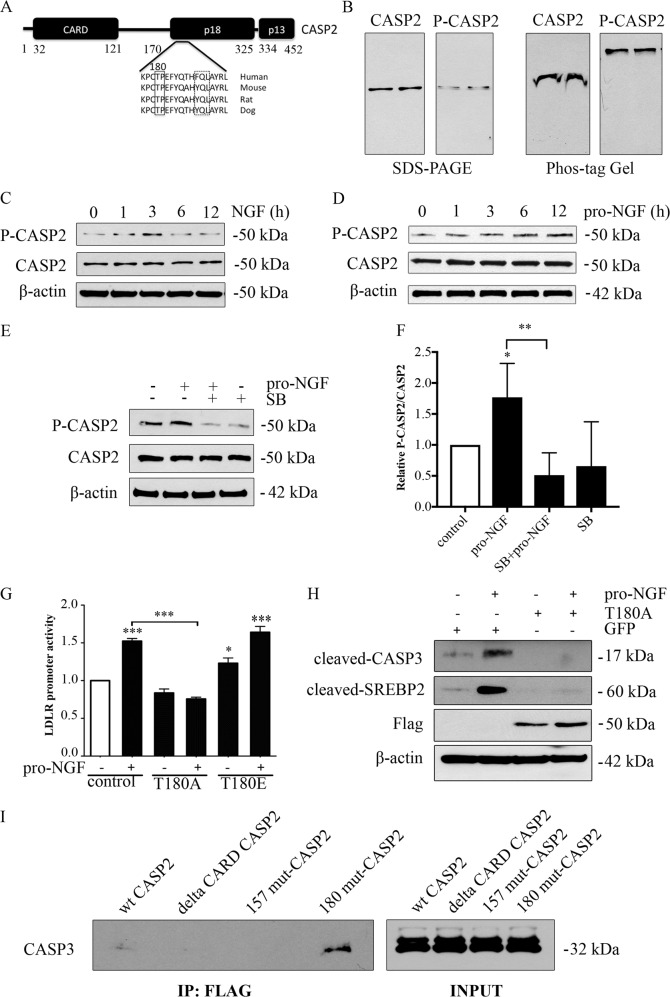
Fig. 3. Role of Caspase-2 in p75NTR mediated regulation of SREBP.
a Structure of human caspase-2 (CASP2) showing the CARD domain and the 18 and 13 kDa caspase subunits. p38 MAPK phosphorylation site at threonine (Thr)180 in CASP2 is conserved among species. The amino acid numbering underlying functional domains in CASP2 is shown. Boxes represent sequence homology among mammalian species. b Phosphoprotein retardation was performed as described in Materials and methods. A specific phospho-CASP2 antibody against Thr180 was used in conjunction with an antibody against CASP2, recognizing non-phosphorylated form of the enzyme. Phosphorylated CASP2 migrated slower in the Phos-Tag gel (right) as compared with the protein run on denaturating SDS-PAGE (left). c, d Huh7 hepatocyte cells were stimulated with 50 ng NGF (c) or 10 ng/ml pro-NGF (d) for different times followed by immunoblotting using phospho-CASP2 and anti-CASP2 antibodies. β-Actin was used as a control. e, f Cells were treated with pro-NGF for 6 h in the absence or presence 1 μM of the p38 MAPK inhibitor, SB203580 (SB). e Immunoblot and f quantification of p-CASP2 levels. SB reduced the increase in p-CASP2 by pro-NGF. Values are means ± SD, n = 4. *p < 0.05 for pro-NGF vs. control and **p < 0.01 for SB + pro-NGF vs. pro-NGF. g Mutant Flag-Thr180A-CASP2 (T180A) and Flag-Thr180E-CASP2 (T180E) caspase-2 constructs were generated by site-directed mutagenesis and transfected for 24 h into Huh7 cells in conjunction with the LDLR promoter construct to measure gene activity. Control cells expressed EGFP plasmid. Half of the cells was then stimulated with 10 ng/ml pro-NGF for 16 h. Luciferase activity was measured as described and normalized to Renilla readout. Expression of mutant T180A construct inhibited the effect of pro-NGF on LDLR, while the T180E construct increased LDLRs in controls. pro-NGF values are mean ± SD, n = 4. ***p < 0.001 for pro-NGF vs. unstimulated controls, and for T180A + pro-NGF vs. pro-NGF. *p < 0.05 for T180E unstimulated vs. controls. h Cells transfected with control GFP or mutant T180A-CASP2 construct were further stimulated with 10 ng/ml pro-NGF for 16 h. The amount of cleaved caspase-3 (17 kDa band, CASP3) and of SREBP2 (60 kDa band) was induced by pro-NGF and reduced in the presence of T180A. Expression of T180A construct is shown using anti-Flag antibodies. i Cell lysates from wild type and mutant caspase-2-expressing cells were subjected to co-immunoprecipitation (IP) using anti-Flag antibodies as described in Materials and methods. Immunoblotting was done using anti-caspase-3 antibodies. Lane 1, wild-type caspase-2; Lane 2, CARD domain lacking caspase-2 mutant; Lane 3, Ser157A caspase-2 mutant; Lane 4, Thr180A caspase-2 mutant