Table 2.
Optimization of reaction conditions
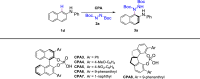
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Cat | Solvent | Temp/°C | Time/h | Yield %a | ee %b |
| 1 | Ph | CPA3 | DCM(D) | −60 | 12 | 63 | 73 |
| 2 | Ph | CPA4 | DCM | −60 | 12 | 24 | 62 |
| 3 | Ph | CPA5 | DCM | −60 | 12 | 75 | 74 |
| 4 | Ph | CPA6 | DCM | −60 | 12 | 94 | 74 |
| 5 | Ph | CPA7 | DCM | −60 | 12 | 58 | 73 |
| 6 | Ph | CPA8 | DCM | −60 | 12 | 11 | 12 |
| 7 | Ph | CPA6 | Toluene | −60 | 12 | 24 | 47 |
| 8 | Ph | CPA6 | Et2O(E) | −60 | 12 | 11 | 41 |
| 9 | Ph | CPA6 | THF | −60 | 12 | <2 | – |
| 10 | Ph | CPA6 | D:E = 1:1 | −60 | 12 | 42 | 88 |
| 11 | Ph | CPA6 | D:E = 7:3 | −60 | 12 | 67 | 90 |
| 12 | Ph | CPA6 | D:E = 7:3 | −70 | 48 | 93 | 91 |
| 13 | Ph | CPA6 | D:E = 7:3 | −78 | 48 | 65 | 92 |
| 14c | Ph | CPA6 | D:E = 7:3 | −70 | 48 | 94 | 91 |
All screening reactions were carried out in a 10 mL glass vial with a PTFE-lined cap on a 0.1 mmol scale. 2.0 equiv of 2a, 10% mol catalyst, 1 mL solvent
aYield represents isolated yield
bDetermined by HPLC analysis
c20 mol% catalyst
