Table 1.
Optimization of resolution of amino phenol 1a
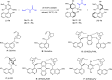
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | 2 | Cat | Solvent | Conv. (%)b | Product, ee (%)c | 1a, ee (%)c | S d |
| 1 | 2a | A | CH2Cl2 | 30 | 3a, 60 | 26 | 5 |
| 2 | 2a | B | CH2Cl2 | 26 | 3a, −26 | −9 | 2 |
| 3 | 2a | C | CH2Cl2 | 76 | 3a, −7 | −23 | 1.4 |
| 4e | 2a | D | CH2Cl2 | 5 | 3a, 73 | 4 | 6.6 |
| 5 | 2a | E | CH2Cl2 | 28 | 3a, 74 | 29 | 9 |
| 6e | 2a | F | CH2Cl2 | 9 | 3a, 78 | 8 | 8.7 |
| 7 | 2a | E | CH3CN | 80 | 3a, 22 | 90 | 4 |
| 8 | 2a | E | THF | 28 | 3a, 20 | 8 | 1.6 |
| 9 | 2a | E | EtOAc | 28 | 3a, 36 | 14 | 2.4 |
| 10 | 2a | E | 1:1 CH2Cl2/CH3CN | 62 | 3a, 50 | 82 | 7 |
| 11f | 2b | E | 1:1 CH2Cl2/CH3CN | 60 | 4a, 65 | 98 | 21 |
| 12g | 2b | E | 1:1 CH2Cl2/CH3CN | 39 | 4a, 86 | 55 | 23 |
aUnless noted otherwise, the reactions were performed with 1a (0.04 mmol, 1.0 equiv.), 2 (0.8 equiv.), catalyst (20 mol%) in solvent (0.5 mL) at 24 oC for 4 h
bDetermined by 1H NMR
cDetermined by chiral HPLC
dS = ln[(1 − Conv.)(1 − ee1a)]/ln[(1 − Conv.)(1 + ee1a)]
e15 h instead of 4 h
fThe reaction time was 24 h
gUse of 10 mol% E and with 24 h reaction time
