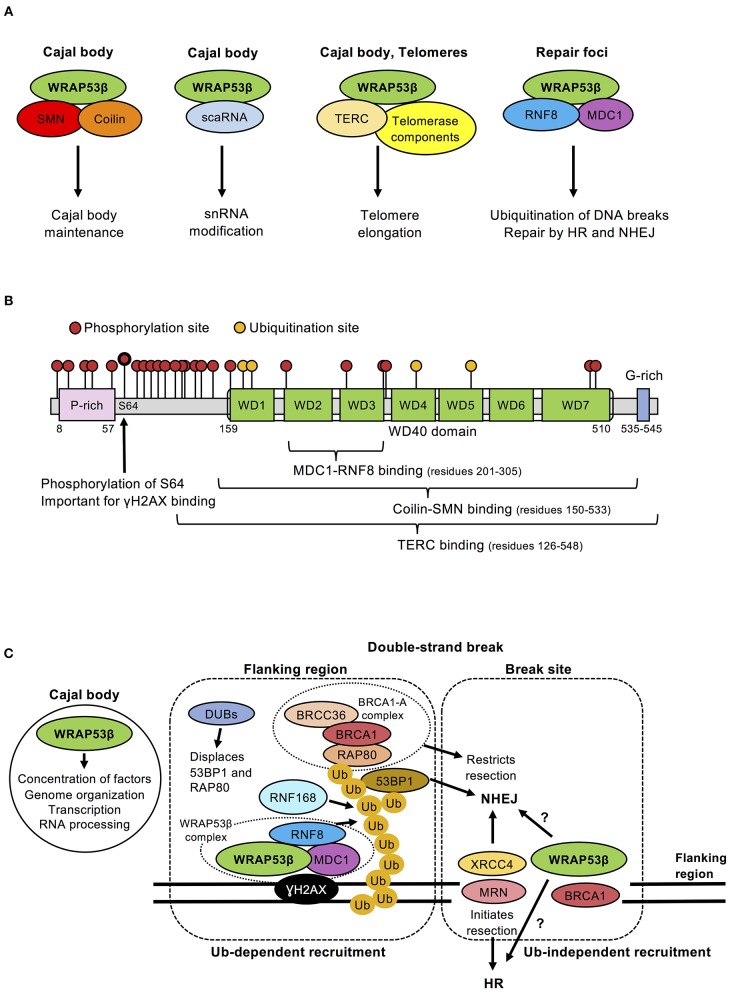
Figure 1.
(A) Schematic illustration of the different WRAP53β complexes, their localization and function. Note: scaRNA and TERC are RNA molecules. (B) Schematic illustration of the domains, binding, phosphorylation and ubiquitination sites in the WRAP53β protein. The sites for post-translational modifications were obtained from PhosphoSitePlus on April 17, 2019. The location of WD40 repeats were predicted using the WD40-repeat protein Structures Predictor (Wu et al., 2012; Wang et al., 2013, 2015; Ma et al., 2019); (WDSP, May 2nd 2019): WD40 1 (amino acid residues 159–197), WD40 2 (residues 207–259), WD40 3 (residues 266–305), WD40 4 (residues 313–354), WD40 5 (residues 358–397), WD40 6 (residues 402–442), WD40 7 (residues 450–510). (C) Schematic view of the functions of WRAP53β in Cajal bodies, at the break site and in surrounding chromatin. Ubiquitin-dependent recruitment of DNA repair factors occurs at regions flanking the break site. WRAP53β binds γH2AX and also scaffolds the interaction between MDC1 and RNF8, which is important for the recruitment of RNF8 to DNA breaks. Once there, RNF8 and RNF168 ubiquitinate proteins at damaged chromatin, which stimulates recruitment of downstream factors 53BP1, RAD51, and BRCA1. BRCA1 forms several sub-complexes with different functions, of which the BRCA1-A complex (containing BRCA1, RAP80, BRCC36, and additional proteins not discussed here) restrict resection. Recruitment to the break site appears to be ubiquitin-independent and the factors recruited here include XRCC4, which promotes NHEJ, or DNA break sensor proteins, such as the MRN complex that promote HR. Pools of WRAP53β and BRCA1 also locate at this site for reasons unknown. Functions performed by WRAP53β in Cajal bodies could potentially be performed at break sites. The recruitment of RAD51, a downstream protein of WRAP53β, to DNA lesions appears to occur via both ubiquitin-dependent and independent mechanisms.