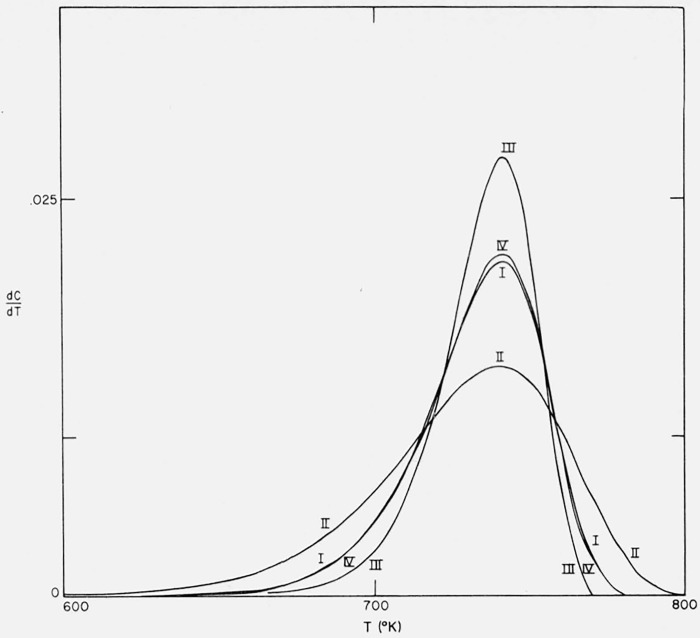
Figure 9.
Effects of activation energy and preexponential temperature dependence on thermogravimetric rate versus temperature.
n = 1
I E = 60,000 cal/mol; A/β = 1016/°K
II E = 40,000 cal/mol; A/β = 9.675 × 109/°K
III E = 80,000 cal/mol; A/β = 9.318 × 1021/°K
IV E = 60,000 cal/mol; A/β = 1.374 × 1013/°K; A(T).