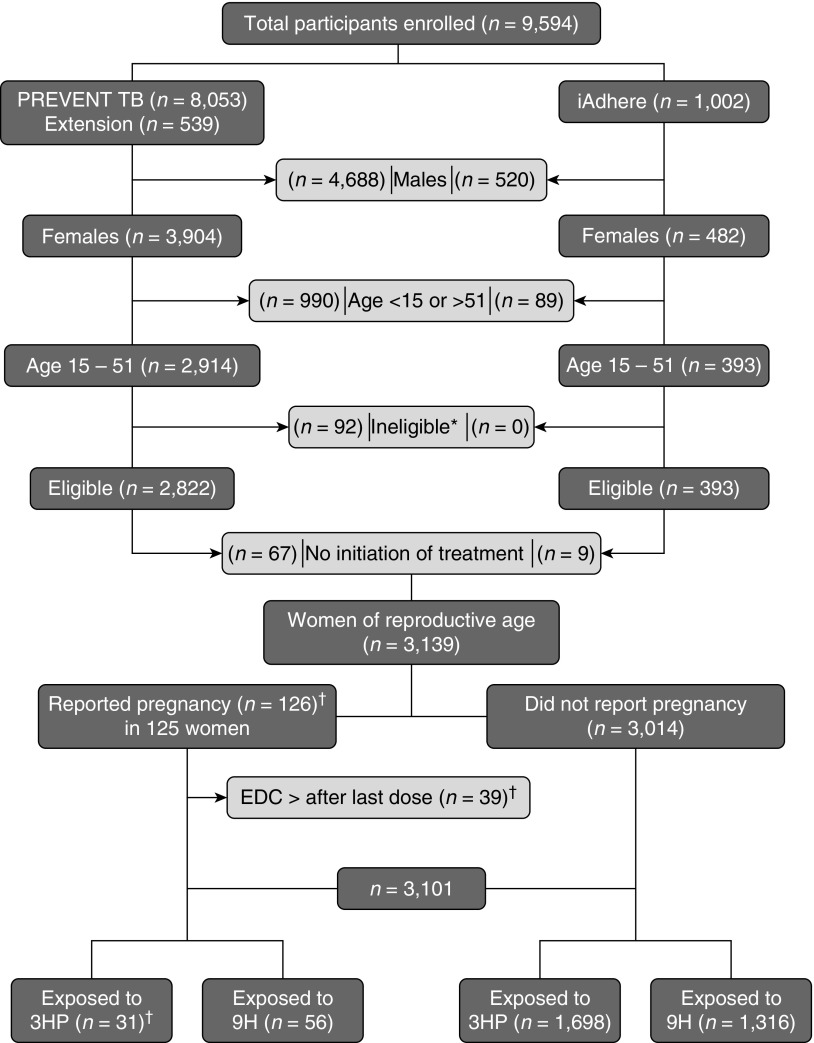
Figure 1.
Pregnancy events safety assessment. This figure shows the total number of participants who were enrolled in the PREVENT TB and the iAdhere trials. Certain groups were excluded to select women of reproductive age who were evaluated in this analysis. In addition, pregnancies in which the estimated date of conception occurred after the last study dose were excluded to identify pregnancies that had been exposed to the study drugs. *Reasons for ineligibility: false positive TST (n = 3), lack of sensitivity test for the index case (n = 4), negative tuberculosis culture of the source (n = 31), isoniazid and rifapentine resistant of the source (n = 53), tuberculosis at enrollment (n = 1). †One participant reported 2 pregnancies (EDC occurred before and after the LD for each pregnancy). 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); EDC = estimated date of conception; LD = last study dose; TST = Tuberculin Skin Test.