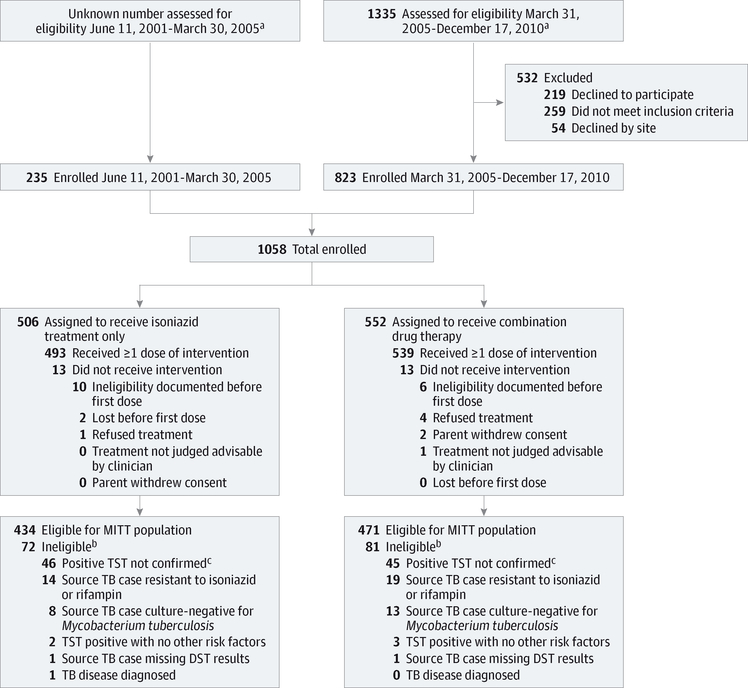
Figure 1. Flowchart of Study Participants (Children Aged 2–17 Years): CONSORT Criteria.
This flowchart shows the number of participants who were enrolled, received the assigned treatment, and were analyzed for the safety and effectiveness outcomes. Combination drug therapy indicates 3 months of directly observed once-weekly combination of rifapentine and isoniazid; isoniazid therapy, 9 months of self-administered daily isoniazid; DST, drug susceptibility testing; MITT, modified intention-to-treat; TB, tuberculosis; TST, tuberculin skin test.
a Eligibility screening data for the randomized clinical trial were obtained from March 31, 2005, onward, with the implementation of an eligibility screening log. This log was implemented in response to the publication of the CONSORT (Consolidated Standards of Reporting Trials) reporting recommendations for randomized clinical trials, which were vetted after the PREVENT TB trial started.
b Enrollment of participants was allowed before Mycobacterium tuberculosis culture and susceptibility data were available in the source case of tuberculosis.
c Results of TST not confirmed as positive on postenrollment TST repeated at 8 to 12 weeks; enrollment of close contacts was allowed if children were younger than 5 years or human immunodeficiency virus seropositive and enrolling clinicians had the option to discontinue treatment.