Extended Data Fig. 5. αPM+HM pseudoislets secrete human insulin upon glucose stimulation in healthy NSG host mice.
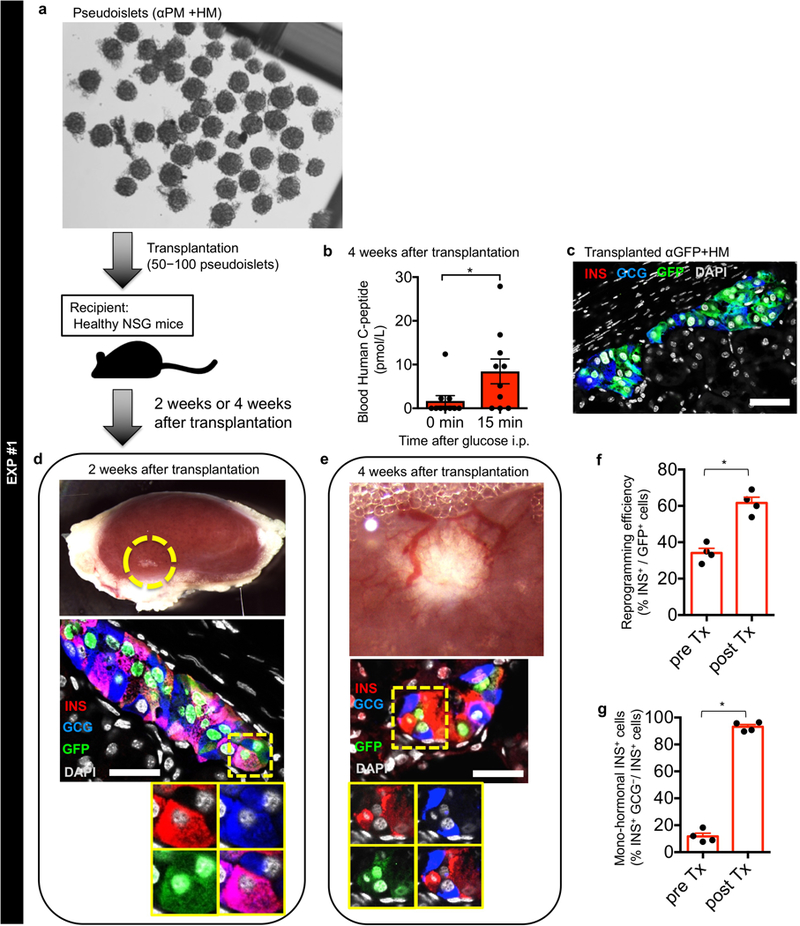
(a) αPM+HM pseudoislets generated from non-diabetic donor samples were transplanted under the kidney capsule of non-diabetic NSG mice. (b) Four weeks after transplantation, in vivo glucose-stimulation tests were performed. Circulating human C-peptide levels are higher after glucose injection compared to before the injection. *p = 0.031, Mann-Whitney test, two-tailed. n = 10 from 6 different donors. (c) Immunostaining picture of control αGFP+HM grafts 4 weeks after transplantation under the kidney capsule of host NSG mice; there is almost no reprogramming into insulin production (< 1%). (d—g) αPM+HM grafts show vascular formation around the grafts and retain abundant GFP-labeled insulin-expressing cells two weeks (d) and 4 weeks (e) after transplantation. Interestingly, αPM+HM grafts display increased reprogramming efficiency (% of insulin+ cells out of GFP+ cells; e, f) and fractions of monohormonal INS+ cell (4 weeks after transplantation; e, g). *p = 0.029, Mann-Whitney test, two-tailed. n = 4 from different donors. All data shown are means ± s.e.m. Scale bars: 25 μm. All images are representative from 6 (a), 3 (c), 2 (d) or 4 (e) different donors.
