Abstract
Background
The triglyceride-glucose index (TyG index) is a tool for insulin resistance evaluation, however, little is known about its association with coronary artery disease (CAD), which is the major cardiovascular death cause, and what factors may be associated with TyG index.
Objective
To evaluate the association between the TyG index and the prevalence of CAD phases, as well as cardiovascular risk factors.
Methods
The baseline data of patients in secondary care in cardiology from Brazilian Cardioprotective Nutritional Program Trial (BALANCE Program Trial) were analyzed. Anthropometric, clinical, socio-demographic and food consumption data were collected by trained professionals. The TyG index was calculated by the formula: Ln (fasting triglycerides (mg/dl) × fasting blood glucose (mg/dl)/2) and regression models were used to evaluate the associations.
Results
We evaluated 2330 patients, which the majority was male (58.1%) and elderly (62.1%). The prevalence of symptomatic CAD was 1.16 times higher in patients classified in the last tertile of the TyG index (9.9 ± 0.5) compared to those in the first tertile (8.3 ± 0.3). Cardiometabolic risk factors were associated with TyG index, with the highlight for higher carbohydrate and lower lipid consumption in relation to recommendations that reduced the chance of being in the last TyG index tertile.
Conclusion
The TyG index was positively associated with a higher prevalence of symptomatic CAD, with metabolic and behavioral risk factors, and could be used as a marker for atherosclerosis.
Trial registration ClinicalTrials.gov identifier: NCT01620398. Registered 15 June, 2012
Electronic supplementary material
The online version of this article (10.1186/s12933-019-0893-2) contains supplementary material, which is available to authorized users.
Keywords: Cardiovascular diseases, Coronary artery disease, Risk factors, Secondary care, Triglyceride-glucose index
Background
Cardiovascular diseases (CVD) are a class of chronic non-communicable diseases (NCD) with the highest incidence of premature death [1] and in Brazil the CVD mortality rate is among the highest in Latin American [2]. In 2015, coronary artery disease (CAD) led to the death of 7.4 million people in the world, being one of the main causes of cardiovascular-related death [3]. The CAD occurs when the coronary arteries are blocked due to the atherosclerosis process, impairment the supply of blood to the heart muscle, which develops slowly and oftentimes, without symptoms [3, 4]. The main manifestation in its symptomatic phase is angina defined as chest pain radiating to the shoulders, arms, and jaw [5, 6].
Type 2 diabetes mellitus (T2DM) is one of the risk factors for CAD and other circulatory system diseases related to the progression and rupture of atherosclerotic plaques [7]. T2DM commonly coexist with artery hypertension and dyslipidemias, known for being endothelial aggressors [8, 9]. On the other hand, food consumption is one of the modifiable risk factors for NCD which can also influence clinical variables.
In its turn, insulin resistance (IR) contributes to the initiation of NCD, playing a key role in the development of T2DM and atherosclerosis [10]. In this sense, the triglyceride-glucose index (TyG index) has been proposed as an alternative method to evaluate IR based on only two parameters, glycemia (mg/dl) and fasting triglycerides (TG) (mg/dl) [11]. Cross-sectional studies show association between TyG index, hypertension [12], artery stiffness and coronary artery calcification [13–15]. Furthermore, contradictory results have been found in longitudinal studies. Studies have been show an association of TyG index with the incidence of CVD [16], coronary artery stenosis [17], stroke [18] and carotid atherosclerosis [19], however, no association was found for the incidence of CAD [20].
In this sense, studies that evaluate the relationship between TyG index, the prevalence of CAD and its association with CVD risk factors are scarce. Furthermore, these associations have not been studied in patients undergoing secondary care, especially among Brazilians. In view of the above, the purpose of this study was to evaluate the association between TyG index, CAD and risk factors for CVD.
Methods
The data analyzed in this study was the baseline of the multicenter study: Brazilian Cardioprotective Nutritional Program Trial (BALANCE Program Trial) registered on ClinicalTrials.gov (NCT01620398) and coordinated by the hospital, Hospital do Coração (HCor), as part of the program “Hospitals with excellent SUS service from the “Programa de Apoio ao Desenvolvimento Institucional do Sistema Único de Saúde (PROADI-SUS)”, in partnership with the Ministry of Health of Brazil.
Patients
Adult and elderly patients of both sexes, aged 45 years or older, who had at least one CVD in the last 10 years, were included. Patients with asymptomatic CAD were considered those that had coronary angiography or coronary angiotomography showed atherosclerotic stenosis ≥ 70% of the diameter of any coronary artery. Patients with symptomatic CAD were those with a history of angina: clinical diagnosis including diagnosis without complementary tests; the history of the positive stress test and patients with treated CAD were those with angioplasty/stent/revascularization.
All eligibility criteria are reported in the study protocol [21]. The patients signed an informed consent form before participating in the study. Note that the study was a multicenter study, therefore each center submitted its study protocol to the local Ethics Committee. The study started only after all the protocols of the centers were approved [21]. The study protocol was developed in compliance with Brazilian and international ethical principles [22].
Data collection
Information on socioeconomic conditions (sex, age, income, level of education), behavior (food consumption, physical activity and smoking), history of diseases and medication use were obtained through structured questionnaires applied by trained interviewers. Weight, height and waist circumference (WC) (midpoint between the inferior border of the costal arch and the iliac crest in the mid axillary line) [23] were measured by trained professionals. The mean of the two WC measures (difference < 1 cm between measures) was the official waist circumference measure. Body mass index (BMI) was calculated using weight (kg)/height (m2). The waist–height ratio was calculated with WC (cm)/height (cm) [24]. Visceral adiposity index (VAI) was calculated by the following formulas: men = [WC (cm)/(39.69 + 1.88 × BMI (kg/m2))] × (TG (mmol/l)/1.03) × (1.31/HDL (mmol/l)); women = [WC (cm)/(36.58 + 1.89 × BMI (kg/m2))] × (TG (mmol/l)/0.81) × (1.52/HDL (mmol/l)) [25]. Blood pressure was measured one time by a trained nurse, with the mercury sphygmomanometer, following the recommendations of the American Heart Association [26].
Blood samples were collected after 12–14 h of fasting. Classical cardiovascular risk markers, such as glycemia, triglycerides (TG), total cholesterol (TC) and high-density lipoprotein (HDL-C), were measured by the enzymatic calorimetric method (Johnsons & Johnsons, Raritan, USA, VITROS 5600). Low-density lipoprotein (LDL-C) was determined by the Friedewald formula. TyG index was calculated by the formula Ln [fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2] [11].
Energy intake (kcal), carbohydrates (g), lipids (g) and protein (g) were evaluated through mean food consumption recorded by two 24-h dietary recalls. Quantitative analysis of the foods was conducted using the Nutriquanti® software. The data collected were the same in all the centers participants in this study.
Statistical analysis
The normality of the data was evaluated by the Kolmogorov–Smirnov test. The data were presented as median (interquartile range) or mean (standard deviation). Comparisons between groups were assessed by the Kruskal–Wallis test and Mann–Whitney U test. The categorical variables were evaluated by the Chi square test for linear trend. The associations between TyG index and the cardiovascular risk factors were obtained through linear regression or multinomial logistic regression (food consumption). Associations between TyG index and subtypes of CAD were obtained by Poisson regression and adjusted by possible confounders. The analysis was performed using SPSS v. 23 for Windows (SPSS, Inc., Chicago, IL, USA) and STATA® 13.0. For all analyses, variations were considered statistically significant for α < 0.05.
Results
A total of 2330 patients underwent secondary care for CVD, with a mean age of 63.2 ± 8.9 years, mean BMI of 29.1 ± 5.0 kg/m2 and 58.1% male.
The most prevalent CVD was treated CAD (69.0%), followed by symptomatic CAD (36.2%) and asymptomatic CAD (16.3%). Other CVD, including peripheral artery disease and stroke, corresponded respectively to 11.2 and 12.0% of total CVD.
Regarding the history of diseases, 90.3% had artery hypertension, 44.0% were diabetic and 78.2% were dyslipidemic. Furthermore, 65.1% of the included patients had a family history of CAD, 90.4%, 94.8%, 41.2% and 86.2% used anti-coagulant, antihypertensives, hypoglycemic and lipid-lowing medication, respectively.
The patients were stratified according to TyG index tertiles. Patients in the last TyG index tertile presented higher values of weight, BMI, WC, waist–height ratio, VAI, systolic blood pressure (SBP), diastolic blood pressure (DBP), TG, glycemia, total cholesterol, LDL-C, and LDL-C/HDL-C ratio compared to the first tertile of TyG index. Also, lower values of HDL-C and age were observed in those in the last tertile of TyG index. In addition, patients who were sedentary, dyslipidemic, diabetic, and hypertensive were more present in the third tertile of TyG index. Carbohydrate intake was higher in tertiles 1 and 2 compared to tertile 3, while lipid intake was higher in the third tertile than in the first tertile (Table 1).
Table 1.
Socio-demographic, anthropometric, clinical and food consumption characteristics of patients in secondary care for cardiovascular diseases according to TyG index tertiles
| Variables | TyG index tertiles | P-value | ||
|---|---|---|---|---|
| 1 (lowest) (n = 777) | T2 (n = 777) | 3 (highest) (n = 776) | ||
| TyG index | 8.3 ± 0.3a | 8.9 ± 0.1b | 9.7 ± 0.5c | < 0.001 |
| Male, sex [%] | 468 [34.5] | 424 [31.2] | 466 [34.3] | 0.942 |
| Age (years) | 63.7 ± 8.8a | 63.4 ± 8.8a | 62.4 ± 9.2b | 0.008 |
| BMI (kg/m2) | 27.5 ± 4.6a | 29.4 ± 5.0b | 30.3 ± 4.9c | < 0.001 |
| Waist circumference (cm) | 95.9 ± 11.9a | 100.2 ± 11.9b | 103.2 ± 11.6c | < 0.001 |
| Waist-to-height ratio | 0.6 ± 0.1a | 0.6 ± 0.1b | 0.6 ± 0.1c | < 0.001 |
| VAI | 1.4 ± 0.7a | 2.5 ± 0.9b | 5.1 ± 4.3c | < 0.001 |
| SBP (mmHg) | 129.0 ± 19.7a | 131.8 ± 19.4b | 131.4 ± 19.4b | < 0.010 |
| DBP (mmHg) | 77.9 ± 12.5a | 79.5 ± 12.4b | 80.4 ± 12.6b | < 0.001 |
| Total cholesterol (mg/dl) | 155.1 ± 39.0a | 170.3 ± 43.3b | 185.3 ± 49.4c | < 0.001 |
| HDL-C (mg/dl) | 47.2 ± 14.3a | 43.0 ± 11.3b | 39.9 ± 9.8c | < 0.001 |
| LDL-C (mg/dl) | 89.3 ± 33.8a | 98.6 ± 38.8b | 97.5 ± 42.5b | < 0.001 |
| LDL-C/HDL-C ratio | 2.0 ± 0.9a | 2.4 ± 0.9b | 2.6 ± 1.2b | < 0.001 |
| Triglycerides (mg/dl) | 89.9 ± 21.7a | 145.0 ± 32.2b | 258.2 ± 154.2c | < 0.001 |
| Glycemia (mg/dl) | 95.8 ± 15.6a | 108.0 ± 24.5b | 151.7 ± 67.6c | < 0.001 |
| Smoking [%] | 475 [33.2] | 453 [31.7] | 501 [35.1] | 0.146 |
| Sedentary [%] | 479 [32.2]a | 493 [33.1]a | 516 [34.7]b | 0.010 |
| Dyslipidemic [%] | 571 [31.4]a | 589 [32.3]a | 661 [36.3]b | < 0.001 |
| Diabetics [%] | 231 [22.5]a | 291 [28.4]b | 504 [49.1]c | < 0.001 |
| Hypertensive [%] | 676 [32.1]a | 709 [33.7]b | 719 [34.2]b | < 0.001 |
| Use of hypoglycemic agents [%] | 205 [21.3]a | 280 [29.1]b | 476 [49.5]c | < 0.001 |
| Use of lipid-lowing agents [%] | 660 [32.9] | 679 [33.8] | 669 [33.3] | 0.468 |
| Family history of CAD [%] | 489 [62.9] | 510 [65.8] | 517 [67.1] | 0.089 |
| Calories (Kcal) | 1437.5 ± 526.0 | 1401.2 ± 497.1 | 1429.3 ± 566.4 | 0.551 |
| Carbohydrates (g) | 189.1 ± 39.1a | 188.5 ± 35.6a | 183.8 ± 39.1b | 0.033 |
| Proteins (g) | 70.5 ± 22.6 | 69.60 ± 20.6 | 71.5 ± 22.5 | 0.329 |
| Lipids (g) | 43.6 ± 12.9a | 44.7 ± 11.9ab | 45.7 ± 12.8b | 0.002 |
Data are mean ± SD (standard deviation) or number [%]. P-values based in Kruskal–Wallis and Mann–Whitney U for quantitative variables and Chi square of linear trend for categorical variables
Different letters show presence of difference and equal letters show the absence of differences
Italic values show the presence of statistic significance
DAP diastolic blood pressure, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein, TyG index triglyceride-glucose index, SBP systolic blood pressure, VAI visceral adiposity index
We assessed the association between TyG index and cardiometabolic risk factors, and found that weight, BMI, WC, waist–height ratio, VAI, SBP, DBP, total cholesterol, LDL-C and LDL-C/HDL-C ratio are positively associated with TyG index, as well as the presence of dyslipidemia, diabetes, hypertension, physical inactivity and smoking. On the other hand, TyG index showed an inverse association with age and HDL-C (Additional file 1: Table S1). These results are independent of sex and medication use.
Also, food consumption was associated with TyG index. Interestingly, carbohydrate consumption above 65% of energy reduces the chance of being in the highest tertile of TyG index by 47%. On the other hand, lipid consumption below 25% of energy is associated with a 27% decrease in the chance of being in the third tertile of TyG, regardless of sex, age and use of medication (Table 2).
Table 2.
Association between TyG index (dependent variable) with energy and macronutrient consumption in patients with heart disease
| Consumption (% energy) | Model 1: TyG index tertiles | Model 2: TyG index tertiles | ||||
|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 (highest) | 1 (lowest) | 2 | 3 (highest) | |
| OR (95% CI) | OR (95% CI) | |||||
| Energy | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 1.0 (0.9–1.0) | ||
| Carbohydrates (%) | ||||||
| 45–65 | 1 (Ref.) | 1 (Ref.) | ||||
| < 45 | 0.9 (0.7–1.3) | 1.1 (0.9–1.4) | 0.9 (0.7–1.3) | 1.1 (0.8–1.4) | ||
| > 65 | 0.7 (0.5–1.1) | 0.5 (0.3–0.7) | 0.8 (0.5–1.1) | 0.5 (0.3–0.8) | ||
| Proteins (%) | ||||||
| 15–20 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||
| < 15 | 0.9 (0.7–1.3) | 0.9 (0.7–1.3) | 1.0 (0.8–1.3) | 1.0 (0.7–1.4) | ||
| > 20 | 0.9 (0.8–1.2) | 1.2 (0.9–1.4) | 1.0 (0.8–1.3) | 1.2 (0.9–1.5) | ||
| Lipids (%) | ||||||
| 25–35 | 1 (Ref.) | 1 (Ref.) | ||||
| < 25 | 0.8 (0.6–0.9) | 0.6 (0.5–0.8) | 0.8 (0.6–1.0) | 0.7 (0.5–0.9) | ||
| > 35 | 0.8 (0.6–1.2) | 0.9 (0.7–1.2) | 0.8 (0.6–1.2) | 0.8 (0.6–1.1) | ||
Data are odds ratio (95% CI) based in multinomial logistic regression
Italic values show the presence of statistic significance
Model 1: crude
Model 2: adjusted by sex, age, use of hypoglycemic, antihypertensive, anticoagulant and lipid-lowing agents
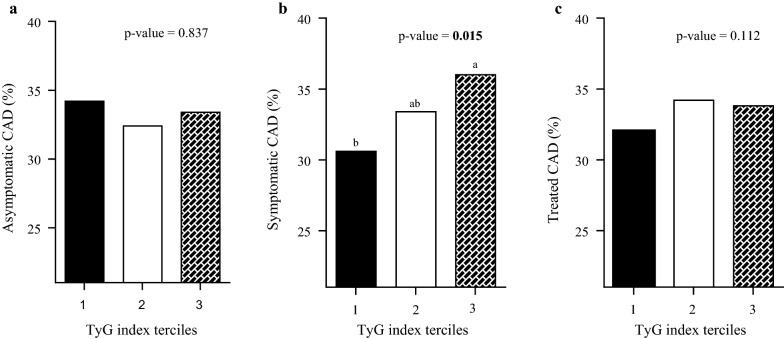
It was found that among the CVD, the prevalence of symptomatic CAD was significantly higher in the third tertile of TyG index compared to the first tertile (Fig. 1). For the other CVD (peripheral artery disease, stroke and heart attack), no differences were found among the TyG index tertiles (data not shown).
Fig. 1.
Prevalence of patients with coronary artery disease (CAD) in the phases a asymptomatic, b symptomatic and c treated according to TyG index tertiles
In fact, the third TyG index tertile presented a significant association with the higher prevalence of symptomatic CAD independent of the influence of social variables (sex, age), lifestyle (smoking, physical activity), clinical history (dyslipidemias, hypertension, diabetes, family history of CAD, use of medication, the respective CVD) and food consumption (calories, carbohydrates and lipids) (Table 3).
Table 3.
Association of TyG index with the different phases of coronary artery disease
| Coronary artery disease (n = 1966) | TyG index tertiles | ||
|---|---|---|---|
| 1 (lowest) | 2 | 3 (highest) | |
| PR (95% CI) | |||
| Asymptomatic (n = 380) | |||
| Model 1 | Ref. | 0.94 (0.75–1.18) | 0.97 (0.78–1.22) |
| Model 2 | Ref. | 0.93 (0.74–1.17) | 0.98 (0.79–1.23) |
| Model 3 | Ref. | 0.93 (0.74–1.17) | 0.98 (0.78–1.17) |
| Symptomatic (n = 844) | |||
| Model 1 | Ref. | 1.09 (0.95–1.25) | 1.17 (1.03–1.34) |
| Model 2 | Ref. | 1.09 (0.95–1.25) | 1.17 (1.03–1.35) |
| Model 3 | Ref. | 1.08 (0.94–1.24) | 1.16 (1.01–1.33) |
| Treated (n = 1607) | |||
| Model 1 | Ref. | 1.07 (1.01–1.14) | 1.05 (0.98–1.13) |
| Model 2 | Ref. | 1.07 (1.01–1.15) | 1.05 (0.98–1.12) |
| Model 3 | Ref. | 1.05 (0.99–1.13) | 1.03 (0.97–1.10) |
Data are prevalence ratio (95% CI) based in Poisson regression
Italic values show the presence of statistic significance
Model 1: crude
Model 2: adjusted by sex and age
Model 3: adjusted by model 2, use of hypoglycemic, antihypertensive, anticoagulant, lipid-lowing agents, carbohydrate and lipids intake, stroke, peripheral artery disease, and the presence of any other stage of the disease
Discussion
The present study investigated the relationship of TyG index with CVD and also with cardiometabolic risk factors in patients with CVD. To the best of our knowledge, this is the first study to associate TyG index with CAD in the symptomatic phase, independent of social, clinical and food consumption characteristics.
The higher prevalence of patients with symptomatic CAD in the highest TyG index tertile suggests metabolic impairment at the symptomatic stage of the disease. The association of TyG index with CAD may be related to atherosclerosis and comorbidities such as dyslipidemias, diabetes and hypertension, among others.
In clinical practice, TG and glycemia are among the classic markers of cardiometabolic risk. Alteration in the levels of these markers are directly associated with IR, progression of atherosclerosis and genesis of CVD. Despite this, few studies have explored the relationship between TyG index and CVD as well as possible influencing factors. Studies have shown that individuals in the higher TyG index quartiles were more likely to have artery stiffness [13, 27] and coronary artery calcification [15, 28] compared to those in the lower quartile. Artery stiffness and calcification of the coronary artery are processes involved in the formation and progression of atherosclerotic plaque [6], and are associated with TyG index, corroborating with our findings. Moreover, studies show an association between the highest values of TyG index, the incidence of hypertension [29, 30], T2DM [31], CVD [16, 32], subtypes of CVD such as stroke [18], as well as events due to the formation of atheroma plaque such as coronary artery stenosis [17]. Also, a study showed that the TyG index is associated with the risk of CVD development compared to the usual tool for IR evaluation [33].
In addition, we observed a positive association between TyG index and risk factors for CVD. In this sense, we showed that WC, BMI, waist–height ratio, VAI, total cholesterol, LDL-C, LDL–HDL ratio, SBP, DBP, smoking, physical inactivity, presence of hypertension and diabetes may influence TyG index. In fact, a positive association of these markers with TyG index is expected due to the compromised metabolic profile of the patients. In this context a study showed that presence of metabolic syndrome, which is characteristic of patients with metabolic impairment profile and risk factor for news diseases can be predictive of future CAD events [34]. Also, the overweight is a risk factor for CVD [35], and we showed that the BMI is associated with the TyG index, which is associated with CAD [36].
Although dietary components are considerable risk factors for CVD, to date no study has evaluated the influence of dietary intake on TyG index. Interestingly, we found that carbohydrate consumption above 65% of energy compared to current recommendation of 45 to 65% of energy, reduced the chance of being in the third tertile of TyG index, while lipid consumption < 25% of energy reduced the chance of being in the last tertile of TyG index, regardless of sex, age and medication use.
Both macronutrients when consumed in excess are associated with increased serum TG [37]; however, we found that consumption below the recommended lipid level and above the recommended carbohydrate level reduced the chances of being ranked in the highest tertile of TyG index. Current evidence suggests that in addition to quantity, the quality of lipids and carbohydrates consumed is associated with the risk of developing CVD. A meta-analysis with a high degree of evidence demonstrated that the partial substitution of saturated fatty acids with polyunsaturated fatty acids, during a minimum of 2 years, reduced the risk of cardiovascular events by 17% [38]. In addition, the substitution of saturated fats with unsaturated fats is associated with the reduced incidence of NCD [39, 40]. On the other hand, the substitution of saturated fats with carbohydrates exacerbated dyslipidemia, a risk factor for CVD [41].
We showed that carbohydrate consumption > 65% of energy reduced the chances of being in the highest TyG index tertile. A recent cohort involving 18 countries showed that carbohydrate consumption > 65% of energy was associated with a higher incidence of total mortality and mortality related to non-cardiovascular causes compared to 45–65% energy, however, the quality of carbohydrate intake was not addressed in this study [42]. The quality of carbohydrates is also associated with the development of NCD [43], such as added sugars and fructose, present mainly in industrial foods, and low fiber [44]. Studies have shown that consumption of a whole-grain diet is associated with reduced risk of CVD, cancers and total mortality [45]. In a randomized 8-week clinical trial, overweight individuals who consumed whole grains had a significant reduction in blood pressure compared to those who consumed diet based on refined grains [46]. Considering the metabolic impairment of the patients included in this study and its related risk factor, dietary intake is associated with comorbidities. Studies show that Brazilians do not consume sufficient vegetables, this trend is higher among those who present higher consumption of ultra-processed foods. Moreover, high (above recommended value) consumption of sugar, saturated fat and sodium and low (below recommended value) consumption of fiber, legumes, fruits and vegetables have been observed [47]. Carbohydrate intake above the recommended value decreases the chances of being in the third TyG index tertile, however this finding should be interpreted with caution, since the prevalence of symptomatic CAD was higher in the third tertile of TyG index compared to the first tertile.
The strength of the present study lies in it being a multicenter study which evaluated a large number of patients with CVD in all regions of Brazil. We evaluated patients with diverse eating habits despite being from the same country, and the food consumption analysis was conducted with a software which prioritized national food composition tables. However, our study has limitations. The patients were categorized as having more than one stage of CAD, however the regression model was adjusted with the presence of phases of the disease. In addition, the quality of carbohydrates, proteins and lipids were not evaluated, instead we conducted a general analysis of macronutrients.
Conclusion
TyG index was positively associated with the higher prevalence of symptomatic CAD, regardless of social, clinical and behavioral characteristics. In addition, metabolic and behavioral risk factors are positively associated with TyG index, where carbohydrate and lipid intake, above and below recommended values respectively, decreases the chances of being at the highest TyG index tertile.
Based on the findings of this study, high TyG index is associated with symptomatic CAD, thus can serve as a complement test for the screening of patients with heart disease and an indication for therapeutic measures. Future studies should evaluate the influence of dietary components on TyG index, especially the quality of ingested macronutrients in order to elucidate the related associations.
Additional file
Additional file 1: Table S1. Association between the TyG index and cardiovascular risk factors.
Acknowledgements
We thank all the patients for participating in this project, the researchers BW, ACBF, CRT, and all participating centers.
Abbreviations
- CVD
cardiovascular diseases
- NCD
non-communicable chronic diseases
- CAD
coronary artery disease
- T2DM
type 2 diabetes mellitus
- IR
insulin resistance
- TyG index
triglyceride-glucose index
- TG
triglycerides
- HCor
Hospital do Coração
- PROADI-SUS
Programa de Apoio ao Desenvolvimento Institucional do Sistema Único de Saúde
- WC
waist circumference
- BMI
body mass index
- VAI
visceral adiposity index
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- SD
standard deviation
- OD
odds ratio
- PR
prevalence ratio
Authors’ contributions
The conception and design of the study were performed by ACBF, CRT and BW. The generation and data collection were performed by AS, ACBF, CRT, BW, APSC, HHMH and JB. The assembly and analysis and/or interpretation of the data were performed by AS, APSC, HHMH, and JB. All authors read and approved the final manuscript.
Funding
This study was supported by the Hospital do Coração (HCor) as part of the PROADI-SUS, in partnership with the Brazilian Ministry of Health. This study was also funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Financial Code 001. J Bressan and HHM Hermsdorff are research productivity fellows of CNPq (Ministry of Science and Technology, Brazil).
Availability of data and materials
All authors take responsibility for the integrity of the data and the accuracy of the data analysis. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The patients signed an informed consent form before participating in the study. Each center submitted its study protocol to the local Ethics Committee because the study was a multicenter study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ribeiro ALP, Duncan BB, Brant LCC, Lotufo PA, Mill JG, Barreto SM. Cardiovascular health in Brazil trends and perspectives. Circulation. 2016;133:422–433. doi: 10.1161/CIRCULATIONAHA.114.008727. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt MI, Duncan BB, e Silva GA, Menezes AM, Monteiro CA, Barreto SM, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377:1949–1961. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Cardiovascular diseases (CVDs). 2017. http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 15 Dec 2018.
- 4.Cagle SD, Cooperstein N. Coronary artery disease: diagnosis and management. Prim Care. 2018;45:45–61. doi: 10.1016/j.pop.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Parmet S. Coronary artery disease. JAMA. 2004;292:2540. doi: 10.1001/jama.292.20.2540. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia Sujata K. Biomaterials for Clinical Applications. New York, NY: Springer New York; 2010. Coronary Artery Disease; pp. 23–49. [Google Scholar]
- 7.Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2016;37:191–204. doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Global Atlas on Cardiovascular disease prevention and control, vol 155. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization [Internet]. 2011: https://apps.who.int/iris/handle/10665/44701. Accessed 15 Dec 2018.
- 9.Introduction. Diabetes care. 2015;38:S1–2. 10.2337/dc15-s001. [DOI] [PubMed]
- 10.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 12.Jian S, Su-Mei N, Xue C, Jie Z, Xue-sen W. Association and interaction between triglyceride–glucose index and obesity on risk of hypertension in middle-aged and elderly adults. Clin Exp Hypertens. 2017;39:732–739. doi: 10.1080/10641963.2017.1324477. [DOI] [PubMed] [Google Scholar]
- 13.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41. doi: 10.1186/s12933-018-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Hear Lung Circ. 2018;27:716–724. doi: 10.1016/j.hlc.2017.05.142. [DOI] [PubMed] [Google Scholar]
- 15.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16:108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 17.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee S-H, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15:155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sánchez-Iñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez J. Risk of incident ischemic stroke according to the metabolic health and obesity states in the Vascular-Metabolic CUN cohort. Int J Stroke. 2017;12:187–191. doi: 10.1177/1747493016672083. [DOI] [PubMed] [Google Scholar]
- 19.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67:665–672. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 20.Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62:345–349. doi: 10.2310/JIM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 21.Weber B, Bersch-Ferreira ÂC, Torreglosa CR, Ross-Fernandes MB, Da Silva JT, Galante AP, et al. The Brazilian Cardioprotective Nutritional Program to reduce events and risk factors in secondary prevention for cardiovascular disease: study protocol (The BALANCE Program Trial) Am Heart J. 2016;171:73–81.e2. doi: 10.1016/j.ahj.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Waist circumference and waist–hip ratio: report of a WHO expert consultation. 2008. https://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf?ua=1. Accessed 1 Nov 2018.
- 24.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 25.Riscimanna ANC. Visceral adiposity index. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AHA. Understanding blood pressure Reading site. 2018. https://www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings. Accessed 1 Nov 2018.
- 27.Won K-B, Park G-M, Lee S-E, Cho I-J, Kim HC, Lee BK, et al. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 2018;17:268. doi: 10.1186/s12944-018-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won K-B, Kim YS, Lee BK, Heo R, Han D, Lee JH, et al. The relationship of insulin resistance estimated by triglyceride glucose index and coronary plaque characteristics. Medicine. 2018;97:e10726. doi: 10.1097/MD.0000000000010726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16:175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, Fernández-Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34:1257–1265. doi: 10.1097/HJH.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc Diabetol. 2017;16:30. doi: 10.1186/s12933-017-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin J-L, Cao Y-X, Wu L-G, You X-D, Guo Y-L, Wu N-Q, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10:6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar J, Bermúdez V, Olivar LC, Torres W, Palmar J, Añez R, et al. Insulin resistance indices and coronary risk in adults from Maracaibo city, Venezuela: a cross sectional study. F1000Research. 2018;7:44. doi: 10.12688/f1000research.13610.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurka MJ, Guo Y, Filipp SL, DeBoer MD. Metabolic syndrome severity is significantly associated with future coronary heart disease in Type 2 diabetes. Cardiovasc Diabetol. 2018;17:17. doi: 10.1186/s12933-017-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González N, Moreno-Villegas Z, González-Bris A, Egido J, Lorenzo Ó. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 2017;16:44. doi: 10.1186/s12933-017-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulten EA, Bittencourt MS, Preston R, Singh A, Romagnolli C, Ghoshhajra B, et al. Obesity, metabolic syndrome and cardiovascular prognosis: from the Partners coronary computed tomography angiography registry. Cardiovasc Diabetol. 2017;16:14. doi: 10.1186/s12933-017-0496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SBC. Atualização da Diretriz Brasileira de dislipidemias e prevenção da aterosclerose—2017. 2017. http://publicacoes.cardiol.br/2014/diretrizes/2017/02_DIRETRIZ_DE_DISLIPIDEMIAS.pdf. Accessed 18 Feb 2019. [DOI] [PubMed]
- 38.Hooper L, Martin N, Abdelhamid A, G DS. Reduction in saturated fat intake for cardiovascular disease (Review). Summary of findings for the main comparison. 2015. [DOI] [PubMed]
- 39.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017 doi: 10.1161/cir.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 40.Billingsley H, Carbone S, Lavie C. Dietary fats and chronic noncommunicable diseases. Nutrients. 2018;10:1385. doi: 10.3390/nu10101385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–509. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390:2050–2062. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 43.Rippe JM, Angelopoulos TJ. Sugars, obesity, and cardiovascular disease: results from recent randomized control trials. Eur J Nutr. 2016;55:45–53. doi: 10.1007/s00394-016-1257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Jaramillo P, Otero J, Camacho PA, Baldeón M, Fornasini M. Reevaluating nutrition as a risk factor for cardio-metabolic diseases. Colomb Medica. 2018;49:175–181. doi: 10.25100/cm.v49i2.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, et al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr. 2016;146:2244–2251. doi: 10.3945/jn.116.230508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.IBGE. Instituto Brasileiro de Geografia e Estatística - Pesquisa de Orçamentos Familiares: 2008–2009. Análise do Consumo Alimentar Pessoal no Brasil. Bibl. do Minist. do Planejamento, Orçamento e Gestão. 2011. http://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf. Accessed 18 Feb 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Association between the TyG index and cardiovascular risk factors.
Data Availability Statement
All authors take responsibility for the integrity of the data and the accuracy of the data analysis. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.