Abstract
Purpose
One of the most common malignancies peculiar to female health with few symptoms, low response to therapy, difficult diagnosis, frequent relapse, and high mortality, is ovarian cancer. Thus, our experiment, using Human amniotic fluid mesenchymal stem cells (hAFMSCs) as a therapeutic tool, aims to find an efficient treatment approach for patients suffering from SKOV3 ovarian cancer.
Material & Methods
In this study, we obtained 5 ml amniotic fluid from 16–20 week pregnant women who underwent amniocentesis for routine prenatal diagnosis by karyotyping in Al‐Zahra Hospital of Tabriz University of Medical Sciences, Iran. Using trans wells in 24 wells plate, hAFMSCs were isolated from all samples, co‐cultured with SKOV3 ovarian cancer cell line, and characterized via flow cytometry and RT‐PCR. Human skin fibroblast cells (HSFCs) were isolated and used as a negative control. SKOV3 and HSFCs' viability after 5 days was evaluated by MTT assay. Cell cycle and apoptotic genes were analyzed by real‐time PCR.
Results
We successfully isolated and characterized hAFMSCs through it positivity for CD44 and CD90 specific mesenchymal stem cell markers and negativity for CD31 and CD45. Oct4 and NANOG were evaluated by RT‐PCR as pluripotency markers, and visualized on 2% gel electrophoresis. We established hAFMS cell lines after 5 days of co‐culturing the SKOV3 cells, viability was decreased; however, HSFCs did not show toxicity by MTT assay. The genes indicated upregulation and high expression by a real‐time PCR.
Conclusions
Our findings showed that hAFMSCs have natural tumor tropism, and can release soluble factors in a cell culture, which cause an efficient anticancer effect. Thus, we can use hAFMSCs for complete anticancer therapy on SKOV3 cell line at cell culture condition and possibly in vivo in the near future.
Keywords: anticancer therapy, apoptotic genes, cell cycle genes, co‐culture, human amniotic fluid mesenchymal stem cells (hAFMSCs), SKOV3
1. INTRODUCTION
Human amniotic fluid mesenchymal stem cells (hAFMSCs) are a unique type of stem cells with high potentials suitable for stem cell‐based therapy for some human life‐threatening disease. These cells can be obtained by amniocentesis in the second trimester or end of pregnancy (Babaei, Gholizadeh Ghaleh Aziz, Alipour, & Naderi, 2018; Kang, Hwang, Kim, et al., 2012). It distinctive characteristics include having high self‐renewal and expression of OCT4, SOX2, PEX1, NANOG, LEFTY2, CRLPTO, and FGF‐4 which fortunately are also self‐renewal and pluripotency markers Kang, Yi, et al. (2012); differentiating capacity of all cells into the three embryonic germ layers; positive for mesenchymal markers such as CD105 (SH2), CD73 (SH3/4), CD90, and CD166, but negative for hematopoietic markers CD31 and CD45 (Gholizadeh‐Ghalehaziz, Farahzadi, Fathi, & Pashaiasl, 2015). Furthermore, hAFMSCs have low immunogenicity, anti‐inflammation and nontumorigenic characteristics. It also maintains its telomere length, forms colony in culture, as well as being kept for many population doublings (more than 250) (Gholizadeh‐Ghaleh Aziz et al., 2017). Its tumor tropism as seen in vivo experiments has opened new perspectives for cancer cell therapies (Kim et al., 2007; You et al., 2015).
Ovarian cancer (OC) is one of the major threats to women's life (McCluggage, 2011). Based on histological category, genotype and molecular pathways, OCs are divided into two groups(Romero & Bast, 2012): low‐ and high‐grade OC. Epithelial ovarian cancer (EOC) as a high‐grade OC is one of the most common and mortal gynecological tumors (McCluggage, 2011; Romero & Bast, 2012). Several molecular pathways link‐up in order to create EOC namely: 1— PI3K pathway. There is (40%) overexpression of genes in this pathway that is associated with BRCA1 or BRCA2 mutations and BRCA1 methylation; 2— Mutations of TP53 (96%); 3— The Rb pathway dysfunction occurs in 67% of high‐grade OCs (Network, 2011; Romero & Bast, 2012). Ovarian Carcinogenesis was created by the interference of 90% P53, up to 40% p21 (WAF‐1), up to 90% cyclin D1 and up to 70% cyclin B1 (Bast, Hennessy, & Mills, 2009; Landen, Birrer, & Sood, 2008; Pashaei‐Asl et al., 2018).
Wild type p53 locus on 17p13.1 has been indicated to be an inhibitor of cancer cells proliferation by controlling cell cycle at Go to G1 passing, G2 checkpoint and G2/M passing (Rahbar et al., 2017). At G1 to S phase inhibition mediated by p21 that stimulated its activities with p53 (Innocente, Abrahamson, Cogswell, & Lee, 1999; Lamb & Crawford, 1986). Therefore, the main function of wild type p53 is to act as a tumor suppressor gene (Baker, Markowitz, Fearon, Willson, & Vogelstein, 1990; El‐Deiry et al., 1993). The most genetic alteration of the p53 gene in human EOC is the mutation on short arm of chromosome 17 that is triggered several modifications of p53 function including acquisition oncogenic function, loss of wild type p53 function or promotion ability to inappropriate activity with no control on cell cycle checkpoints causing unlimited proliferation and growth in cancer cells (Curtin & Spinella, 2005; Wang et al., 2001). P53 mutation is an initial occurrence in ovarian cancer cells. This event is intensely related to high‐grade ovarian neoplasms (Pashaei‐Asl et al., 2018; Skomedal et al., 1997).
P21 (CIP1/WAF1) or CDKN1A (cyclin‐dependent kinase inhibitor 1A) gene is located on chromosome 6p21.2 in humans (Harper, Adami, Wei, Keyomarsi, & Elledge, 1993; Xiong et al., 1993). The activity of this gene is the cell cycle arrest (regulator of cell cycle progression) at G1 and S phase (DNA replication and repair of its damage). P21 function and expression are controlled and mediated by P53 as a tumor suppressor gene (Rodriguez & Meuth, 2006). The WAF1 is downstream of the wild type p53 gene and p53 effects on its promoter region thus are regulated by the cell cycle arrest activity of p21. In tumor cells, P53 gene is mutated so that the normal function of P21 gene is compromised and disturbed (Aziz et al., 2019).
Patients with high‐grade OCs almost do not show any sign and symptoms for discovering tumor, so they are often diagnosed at the last stage of OC with poor prognosis, metastasis, and abdominal implantation occurrence which result in reduction of their quality of life. The 5‐year survival rate of this lethal disease is ~30% (Kim, Ueda, Naka, & Enomoto, 2012). As traditional treatments over the past 30 years, radiotherapy and chemotherapy have been utilized by gynecological oncologists to treat this disease inside tumor origin surgery. However, in the last‐stage cases, metastasis persisted and thereby resulting in death (Lynch et al., 2009).
Cyclin D1 located on chromosome 11q13 is produced through phase G1. It is gathered in the nucleus of a cell and degraded upon the application of its effect in this phase after the commencement of phase S in the cell. This cyclin has the role of mitogen or is a stimulant of mitosis in normal cells cycle. Mitogen growth factors cause the accumulation of cyclin D1 which stimulates Raf and PI‐3K in the subsequent stages and finally causes the progression of mitosis and passing from phase G1 (Aziz et al., 2019). Cyclin D1exists in natural cells at the point of transition of cell from phase G1 to phase S during inspection points for the repair of damaged DNA and prevention of accumulation of mutations. Genetic abnormalities occur in cell cycle in most cancers in phase G1 especially for cyclins (Aziz et al., 2019). The increase in the activity of cyclin D1 in ovarian cancer is probably regulated by steroids, like estrogen. There is a relationship between increase in the expression of this gene and development of ovarian epithelial tumors and the rate of tumor progression, such that, the amount of expression in grade 1 is more than in grade 2 and 3. This shows that the involvement of this gene in the early stages of the formation of epithelial tumor. Live expectancy in these patients is less (Barbieri et al., 2004).
Cyclin B1 is located on chromosome 5q13.2. It is one of the most important proteins in the control of cell cycle which is varies in its amount at different points. It is involved in cell cycle in late stages of phase S and in passing from phase G2 to M. It acts in combination with CDK1 and becomes slippery and finally destroyed after playing its role in cell cycle by ubiquitin‐dependent proteolysis (Norbury & Nurse, 1992). The expression change of this cyclin seems to be connected to the deactivation of gene P53 (Pines & Hunter, 1989). Increased expression of cyclin B1 is seen in early stages of ovarian cancer (Zheng et al., 2009).
Recent studies have proven that cell‐based therapies are promising for cancer treatment. hAFMSC is particularly suitable for this experiment because it can be genetically modified in cancer cells to exert anti‐tumoral effects (Aziz et al., 2019). These cells grow easily in culture environment which have natural tendency to cancer cells (Kang, Hwang, Kim, et al., 2012). Moreover, amniotic fluid is easily available and has no ethical problems associated with fetal and maternal health research (Stuckey & Shah, 2014). In the past decade, hAFMSCs have had major role in antitumoral therapeutic researches including bladder cancer (Chung & Koh, 2013; Stuckey & Shah, 2014), breast cancer (Kang, Hwang, Yi, et al., 2012), and ovarian cancer (Li et al., 2015). Several studies on animal EOC were done in 2015 and confirmed specific antitumoral effect of these cells, hence mesenchymal stem cells from amniotic fluid are acceptable as strong option in gene therapy and tissue repair because the cells have a chromosomal stability and high proliferation (Li et al., 2015; You et al., 2015). In this research, we examine the therapeutic effects of hAFMSCs on Human Exploration and Operations Committee through indirect co‐culturing in vitro to find out the cellular interaction and signaling pathways and effects on cell cycle genes expression by real‐time PCR. Scientists have tried to find critical treatment on EOC with stem cells therapy. In this study, we co‐cultured AFSCs and EOC (SKOV3) to confirm cancer cell therapy by evaluating the expression of P53, P21, cyclinD1, and B1 before and after co‐culturing at Human Exploration and Operations Committee cells.
2. METHODS AND MATERIALS
Experiment procedures were performed under Tabriz University of Medical Sciences' Ethics Committee Guidelines (registered number 5.4.753 at ethic committee of TUMA). After providing written informed consent that was approved by the ethics committee, 5 ml amniotic fluid from pregnant women between 15–20 w gestation, undergoing amniocentesis for routine karyotype screening and skin biopsy (from one person) was obtained from Al‐Zahra hospital; Tabriz University of Medical Sciences.
2.1. Culture and media
2.1.1. Isolation and culture hAFMSCs
hAFMSCs extraction was done in accordance to our previously published research article (Aziz, Pashaei‐Asl, Fardyazar, & Pashaiasl, 2016). Amniotic cells were isolated from the samples by centrifugation at 450 g for 10 min then preserved at 37°C prior to culturing. Cell pellet was re‐suspended in AmnioMAXTM ‐II Complete Medium (Gibco, cat# 11269‐016) in 6‐well plates for two weeks and was incubated in SANYO CO2 Incubator‐ MCo‐19AIC, at 37°C in a 95% humidified chamber with 5% carbon dioxide. Media were changed every three days and nonadherent cells were removed by media exchange. After 2 weeks in primary culture, these cells reached 80%–90% confluency. They were trypsinized by 0.25% Trypsin‐EDTA (1X) (Gibco, cat# 25200‐056) and were plated in T25 cm2 flask. After P1 (passage1), cells were passaged every 7–8 days and stayed in culture for 7 months to reach passage 18–20.
2.1.2. Culture SKOV3
SKOV3 (Human Epithelial Ovarian Cancer Cells, NCBI code: C209) were prepared from Pasteur Institute Cell Line Bank of Iran. These cells were cultured and kept for our study in Dulbecco's Modified Eagle's Medium—low glucose (DMED‐L) Gibco (Germany) supplemented with 10% fetal bovine serum Gibco, 100 U/ml penicillin and 100 µg/ml streptomycin Gibco. Cells were incubated at 37°C in a CO2 incubator with 5% CO2 and 95% air.
2.1.3. Isolation and culture of skin fibroblast cells from skin biopsy
As negative control, human fibroblast cells were utilized. Skin fibroblast cells were isolated via below protocol. This protocol describes the steps for obtaining a primary fibroblast cell line from human skin biopsies. Fibroblasts are derived directly from excised skin as explants. Enzyme digestion by collagenase (Gibco, Cat#17104‐019) may help to obtain cells in a shorter time. First, the skin biopsy is rapidly washed in PBS in a Petri dish, cut into small fragments and transferred to a flask. Then using a sterile Pasteur pipette with flame‐rounded tip, the small tissue fragments are distributed over the bottom surface of the culture flask. The flask was passed rapidly over Bunsen flame in order to evaporate the medium so that the minced tissue pieces adhere to the plastic surface, but also carefully done so as not to heat‐damage the minced tissue. BME medium (Gibco™ Basal Medium Eagle cat#21‐010‐046) (BME) 80 ml, Gibco Fetal bovine serum (FBS) 20 ml and Gibco Penicillin–Streptomycin solution 100× 1 ml) were carefully added for fibroblast growth, then the lid of the flask was firmly closed and placed in CO2 incubator. The culture medium was replaced after 2 days and thereafter replaced three times a week. The fibroblasts started growing from the minced fragments in 2–3 days. When there are sufficient cells, they were detached enzymatically and plated in Petri dishes, or 75 cm2 culture flasks for proliferation. The minced fragments in the flask continued to produce cells for a while. Human skin fibroblast cells (hSFCs) were maintained in DMEM‐L supplemented with 10% FBS and 100 U/ml penicillin and 100 µg/ml streptomycin in CO2 incubator with 5% CO2 and 95% air at 37°C.
2.1.4. Ovarian cancer cell—amniotic fluid stem cell co‐culture
About 40,000 ovarian cancer cells were seeded in 24‐well tissue culture plate by DMEM‐L containing 10% FBS, penicillin (100 U/ml), and streptomycin. After 24 hr, 0.4 µm pore‐sized Transwells™ (ThinCertTM 24 well, greiner bio‐one, 662,640, 0.4 µm translucent RoTrac, PET Membrane) were inserted in to the 24‐well tissue culture plate and hAFMSCs suspensions containing 25,000 cells were seeded in to the Transwells™. Thus hAFMSCs and ovarian cancer cells were co‐cultured indirectly (without physical contact, but cells communication occurred with paracrine signaling via soluble factors) within the same environment (containing medium and factors secreted by the cells) up to 5 days. Medium was changed twice within the period. As negative control, hSFCs were co‐cultured with hAFMSCs with the same protocol.
2.2. MTT assay
After 5 days of co‐culturing, the effect of hAFMSCs on SKOV3 cell line and on hSFCs was evaluated by MTT assay. Three wells in 24‐cell well plates for each concentration were considered to eliminate the sampling error. MTT Powder (Dimethyl Thiazole Diphenyl Bromide, Sigma‐Aldrich) was dissolved in 1x PBS and DMEM‐l supplemented with 10% FBS. About 5 mg/ml of MTT solution was added to co‐cultured plate after which the content of each well of 24‐well plate was transferred to 96‐well plate. The plates were subsequently incubated at 37°C for 4 hr making the purple colored formazan crystals visible. The color is an indication of metabolically dynamic and live cells. Supernatants were discarded and 200 μl of 99% dimethyl sulfoxide (DMSO, Tokyo, Japan) was added to each well to dissolve the formazan crystals. Lastly, the optical density was calculated using a spectrophotometer (with an ELISA plate reader (Awareness, Technology Inc) at two spectrums of 570 and 630 nm where formazan crystals have maximum optical densities. It is considerable that live cells have higher absorption rate. Effects of this co‐culture were further evaluated by statistical analysis.
2.3. RNA extraction and qRT‐PCR
After 5 days of co‐culturing, Transwells™ containing stem cells were discarded. Total RNA of SKOV3 and hSFCs in lower well were extracted using RNX‐Plus kit (CinnaGen, cat# RN7713C) in accordance to its protocol. The complementary DNA (cDNA) was created from extracted RNA using cDNA Synthesis Kit (Fermentas, cat# K1621) about 20 µl for each sample and was performed in a Thermal Cycler (PeQLab). Primers used for qRT‐PCR. As an internal control, it utilized b‐actin (housekeeping gene). mRNA levels of β‐Actin, P53, P21, cyclinD1 and B1 genes were measured in SKOV3 and human fibroblast cells with SYBR® Premix Ex Taq™ II (Tli RNase H Plus) (Ashburner et al., 2000) as qRT‐PCR master mix in a Rotor‐Gene 6000 Real‐time PCR Detection System (Corbett, UK), matching to the manufacturer's instructions. The temperature setting of qRT‐PCR amplification. Relative gene expression was calculated by 2–ΔΔCt method (Livak & Schmittgen, 2001) and was compared with the effects of hAFMSCs on SKOV3 with hSFCs. Furthermore, t test was done for statistical analysis.
3. RESULTS
3.1. Isolation, culture, and morphological observation of hAFMSCs
hAFMSCs were efficiently extracted from 10 amniotic fluid samples. These cells tend to adhere to plastic surface. hAFMSCs in first passage had round shape, colony‐forming growth and on attaining 90% confluency, they showed multilayer growth pattern; although, elongated in the other passages, spindle shaped with monolayer growth pattern. The growth rate in early passages was faster than later ones. (Figure 1a‐c).
Figure 1.
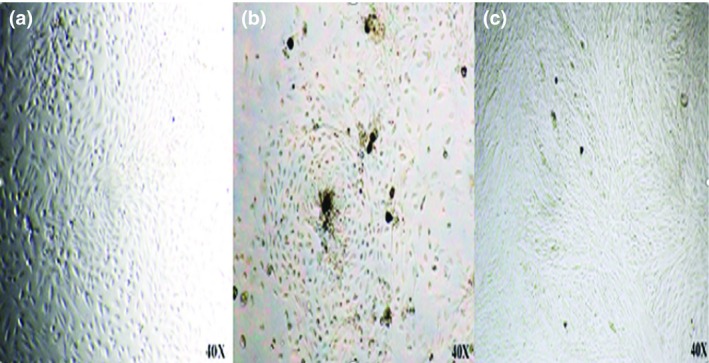
Isolated hAFMSCs in primary passage. (a) Round shape. (b) Colony forming growth. (c) Multilayer growth
3.2. Isolation, culture, and morphological observation of hSFCS
Human skin fibroblast cells (hSFCs) were successfully isolated from skin sample. They had very slow proliferation in the first passage after which it reached 90% confluency in 7 days and became elongated thus forming a very long shape. (Figure 2).
Figure 2.

Human skin fibroblast cells (hSFCs). Spindle shape at cell culture (40× magnification)
3.3. MTT assay
According to our study, hAFMSCs after 5 days of co‐culturing had cytotoxic effect on SKOV3 and suppressed their proliferation. However, it had synergic effect on hSFCs and promoted growth of hSFCs. We calculated the percentage of live cells using the control group with the following formula: Mean optical absorbance of treated cells × 100/Mean optical absorbance of control cells. Percentage of viable cells for SKOV3 was 78% while hSFCs was 129%. These results showed that hAFMSCs had anticancer effects especially on ovarian cancer cells (Figure 3).
Figure 3.
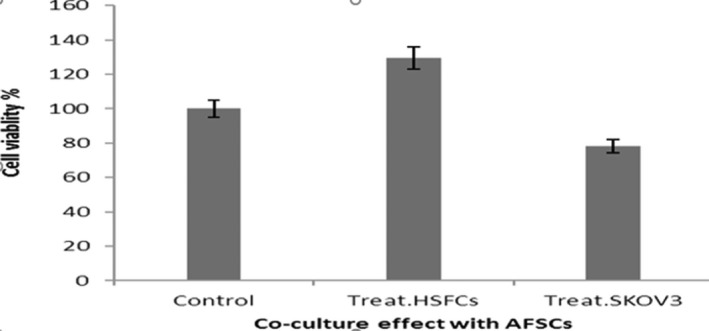
Normalized MTT assay data for co‐culture hAFMSCs with SKOV3 and hSFCs. The cells in co‐culturing state were indicated as control. The results demonstrated cell viability after 5 days of co‐culture with SKOV3 was significantly reduced (p < 0.05) but hSFC did not significantly reduce, it rather was promoted.
3.4. RNA extraction and qRT‐PCR
The expression of β‐Actin, P53, P21, cyclinD1 and B1 genes was studied by quantitative relative real‐time PCR for SKOV3 and fibroblast after co‐culturing with AFSCs (Figure 4). This experiment suggested the induction of apoptotic process in cancer cell by AFSCs but this effect was not seen in fibroblast cells. According to our study, the expression comparison of mRNA in P53, P21, cyclinD1 and B1 between SKOV3 and fibroblast (as control) showed significant promotion for SKOV3 (p < 0.05).
Figure 4.
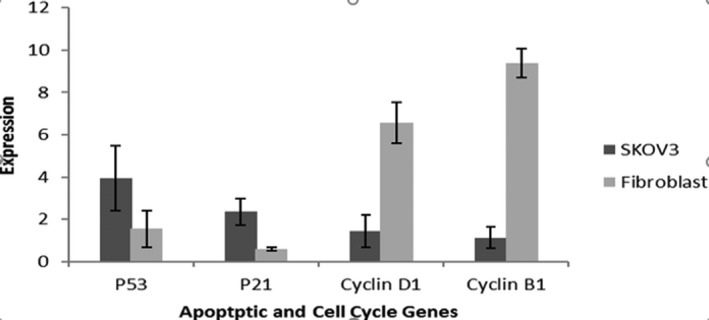
Relative expression of apoptotic and cell cycle genes of SKOV3 and fibroblast (hSFCs) cells after co‐culturing with hAFSCs. Expression level of P53, P21, cyclinD1 and B1 was high at SKOV3 (p < 0.05)
4. DISCUSSION
The anticancer effects of mesenchymal stem cells, in particular human amniotic fluid stem cells, on the gene expression of cancer cells involved in apoptosis have not been clearly studied so far. The majority of studies in recent decade, especially in the field of ovarian cancer, have been done on body of mice and the analysis of the studies was in the form of the target tissue staining and/or evaluation of biological markers.
Therefore, in this study to prove the anticancer properties of mesenchymal stem cells derived from human amniotic fluid stem cells (hAFSCs), we did co‐culturing of ovarian cancer cells with stem cells of amniotic fluid in cell culture (Kang, Hwang, Kim, et al., 2012) using human fibroblasts cells as the negative control. With MTT assay, we also evaluated the survival rate of cancer cells and fibroblasts while the expression amount of P53 and P21 genes was examined via real‐time PCR approach.
Human amniotic fluid stem cells (hAFSCs), according to the results of our study, have anticancer properties; because, the survival rate of cancer cells after co‐culturing is reduced significantly compared to the control group of cancer cells and negative control group (fibroblasts cells).
Scientists, in recent years, have done several researches on the effects of different types of cancer stem cells including Kang et al. in Korea (2012) who worked on therapeutic effects of amniotic epithelial stem cells in breast cancer. In this study, amniotic stem cells were utilized instead of tumor growth in a mouse body; at the same time, the co‐culturing of stem cells and cancer cells was done in the laboratory. According to this study, a significant therapeutic effect without any side effect compared to chemotherapy was reported in mice's body and in culture medium. On the other hand, the growth, invasion, and encroachment of breast tumor were also reduced significantly in therapeutic approach involving amniotic stem cells (Kang, Hwang, Kim, et al., 2012).
According to a study, by using supernatant of growing amniotic stem cells in culture medium, on breast and cervical cancers with apoptosis induction, the survival rate of cells was reduced which shows an increase in the expression of apoptosis‐stimulating proteins including caspase 3 and 8 (Niknejad, Khayat‐Khoei, Peirovi, & Abolghasemi, 2014).
In addition, the study of Kang et al showed more than 50% decrease in the amount of growth and proliferation of cancer cells in a culture medium and also in mice bodies. Evaluating the cytokines involved in this reaction, they pointed out the key roles of TNF‐α, TGF‐β, TNF‐β, and IFN‐γ on anticancer effect. This role of the factors in induction of apoptosis has been confirmed in other studies (Kang, Hwang, Kim, et al., 2012).
Amniotic mesenchymal stem cells play an important role in ceasing the effects of cancer cells up to 30%–50%. An increase in the Bax expression, caspase 3 and 8, on the other hand, a reduction in the Bcl‐2 expression in Glioma cancer cells confirms the activation of apoptosis by mesenchymal stem cells (Jiao et al., 2012).
Therefore, amniotic stem cells cause the induction of its anticancer effects by stimulating the caspase cascade (caspase 3 and 8) and apoptosis‐stimulating factors through induction of internal and external apoptosis pathway. The findings of our study indicate a significant increase in the expression amount of P53 and P21 genes after co‐culturing in cancer cells compared to fibroblast cells. This shows the stimulation of apoptosis pathway, activation of genes responsible for apoptosis as confirmed by findings from previous studies.
When DNA and cell stress are damaged, P53 causes an increase in transcription from genes involved in cell cycle and planned cell death (Shibata‑Kobayashi et al., 2013) including an increase in expression of P21. P21 causes the inhibition of cyclin‐kinase complex (CDK/cyclin). Thus, retinoblastoma protein (Rb) remains and E2F factor is not released consequently, cessation of cell cycle occurs (Jackstadt, Jung, & Hermeking, 2013). In other words, for the inhibition of cell cycle, the expression of P53 and P21 increases (Shibata‑Kobayashi et al., 2013). Our findings also confirm the apoptosis induction in cancer cells by stem cells causes increase in P21 and P53 gene expressions as found in previous studies.
P53 acts as a transcription factor for the family of Peru apoptotic or Bcl‐2 apoptosis activator proteins including Puma, Noxa, Bid and Bax that cause the induction of mitochondrial permeability, the release of cytochrome C and finally the activation of caspases (Tiwari, 2012).
On the other hand, it causes the induction of external pathway proteins including Fas/CD95, DR5, PERP and caspase 6 and 9. Therefore, P53 shows its anticancer behavior on transcription path by induction of apoptosis stimulus factors and antiapoptosis suppression factors in tumor cells (L. Jiang, Sheikh, & Huang, 2010).
P21 is also a negative regulator (inhibitory) of cell cycle which does the direct inhibition of DNA production, kinases function, and indirect inhibition of cyclins dependent to kinase with phosphorylation CDK 1 & 2. The first cell cycle inhibitory activity of P21 starts with inhibiting the CDK2 function. In addition, it is useful in regulating genes transcription including E2F1, E2F1‐dependent Wnt4 inhibition, expression inhibition of STAT3‐dependent genes, MYC‐dependent factors, etc. P21 is involved in moderation of apoptosis, inhibition of cell cycle progression and also connects to PCNA to effect DNA repair via increasing apoptosis in two P53‐dependent/independent mechanisms during DNA repair mechanism. Cells in the G1 phase have rapid growth, synthesis of proteins, DNA precursors, and ribosomes are the key significant processes executed in the G1 phase. CDK enzymes also play a role in the regulation of cell cycle (He et al., 2013). One of CDK inhibitors can be P21 even though P21 acts as an inhibitor in the entire process of cell cycle.
Amniotic mesenchymal cells are involved in secreting soluble factors that halt cell cycle during phase transition from G0 to G1 and prevent the cell from proceeding to the phase S. The evaluation of the expression of cell cycle genes showed that these cells play vital role in the inhibition of positive stimulatory signals to increase the performance of cell cycle and growth of cancer including the inhibition of various cyclins (cyclin D2, cyclin E1, cyclin H), inhibition of the kinases dependent to these cyclins CDK4, CDK6, and CDK2 and finally inhibition of CDKN2B (Magatti, De Munari, Vertua, & Parolini, 2012). On the one hand, with increasing the expression of cyclins G2 and p21 or CDKN1B and p15 or CDKN2A and in the end of increase of the expression, retinoblastoma proteins cause the blocking of cell cycle progression from phase G0 to G1. Findings from our study, with respect to the expression reduction of cyclin D1 and B1 genes which represents inhibition of the cell cycle in the phase change from phase G0 to G1 and inhibition of phase S commencement, corroborates same view from previous studies. This expression reduction of cyclins D1 and B1 after co‐culturing in comparison with the control group of cancer cells and the negative control group (fibroblasts cells) represents inhibition of proliferation and cell cycle activity. According to the directions and stimulation of intracellular signaling mechanisms of apoptosis, P53 and P21 genes caused a reduction in performance and inhibition of cyclins D1 and B1.
In the case of cyclins available in cell cycle, cyclins D1 and B1 were examined in our research, because among the various cyclins, types D1 and B1 are more involved in formation of ovarian epithelial tumor (W. Jiang et al., 1992; Zheng et al., 2009). Cyclins and Cyclin D1‐dependent kinases play a key role in regulation of progression of cell cycle from phase G1 to phase S; therefore, the inhibition of Cyclin D1 causes the nonentrance of cell to phase S. On the contrary, its overexpression causes the shortening of phase G1 and the cell is reproduced faster. Cyclin B1 is involved in the late phase S of the cell cycle and in passing from phase G2 to M. It acts in combination with the cyclin‐dependent kinase1. The expression amount of cyclin genes, therefore, is increased in cancer cells indicating that mitosis is occurring faster (Zheng et al., 2009).
The greatest advantage of mesenchymal stem cells compared to conventional treatments is its antitumoral property, specific implantation in the tumor tissue, ability to transfer several anticancer agents together and simultaneously to the tumor site, as well as stimulation of the immune response (Li et al., 2015).
Among various sources of mesenchymal stem cells, mesenchymal stem cells derived from amniotic fluid have attracted the attention of scientists because of its unique features. These features include having no moral/ethical issues in sample (amniotic fluid) collection, very low cost and easy to extract, very low immunogenic reaction, intermediate capacity, and power of adult and embryonic stem cells, high migration tendency toward tumor cells in vivo, anti‐inflammatory and anticancer effects at tumor site (Li et al., 2015). Furthermore, unlike the other mesenchymal stem cells, Amniotic mesenchymal stem cells do not transform into tumor tissues thus maintaining normal karyotype in the course of culturing even in high passages, long telomere, that is the absence of telomere shortening during high passages (Perin, Sedrakyan, Da, Sacco, & De Filippo, 2008).
In summary, amniotic fluid stem cells with its unique properties in the field of cell therapy for cancer treatment have shown clear anticancer effects in our study with similar observation in previous works. With a variety of mechanisms including induction of internal and external pathways of apoptosis; active involvement of the key role of P53 in regulating and moderating caspases' function and stimulation of apoptotic agents; while on the other hand, stimulation of P21 to regulate the performance of control points of cell cycle such as cyclin D1 and B1 as well as interaction with growth media, amniotic fluid stem cells showed growth inhibitory and anticancer effects.
5. CONCLUSION
Stem cells derived from amniotic fluid are one of the safest and most accessible stem cells. During the past decade,, scientists have tried exploiting its unique properties and functions for research in developing treatment for some of the most chronic and incurable diseases. In the meantime, cancer therapy has gained the highest percentage of studies involving Amniotic mesenchymal stem cells especially, ovarian cancer which often is detected in its advance stages. According to the results of our study, there can be an effective storage of amniotic fluid stem cells for each person during pregnancy in the second quarter (in case they are trisomy screening candidates) or in the third quarter. This might be a very valuable and unique specimen for developing treatment of diseases in newborns and their mothers in the future. Judging from pluripotency of stem cells derived from amniotic fluid and its high capacity to differentiate into several cell categories such as fats and bones, its therapeutic potentials have become more evident. Thus this study has successfully highlighted satisfactory anticancer effects of hAFSCs through effective expression of apoptotic and cell cycle genes with significantly increased P53 and P21 expression and decreased expression of cyclin D1 and B1 in vitro.
CONFLICT OF INTEREST
The authors have no conflicting financial interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors thank the Department of Biochemistry, Faculty of Medicine, Urmia University of Medical Sciences, Department of Molecular Medicine, Faculty of Advanced Medical Sciences of Tabriz University, and Women's Reproductive Health Research Center of Training Center Al‐Zahra Hospital of Tabriz University of Medical Sciences for all support provided (Study ID: 93/4‐10/2).
Gholizadeh‐Ghaleh Aziz S, Fardyazar Z, Pashaiasl M. The human amniotic fluid mesenchymal stem cells therapy on, SKOV3, ovarian cancer cell line. Mol Genet Genomic Med. 2019;7:e726 10.1002/mgg3.726
ENDNOTE
Proliferating Cell Nuclear Antigen.
REFERENCES
- Abolhasani, S. , Gholizadeh Ghaleh Aziz, S. , Khabbazi, A., & Alipour, S., (2018). Determination of the relationship between the severity of Behcet's disease and the expression and methylation of IL‐10, IL‐6 and IL‐8 genes. International Journal of Research in Applied and Basic Medical Sciences, 4(1), 6–14. [Google Scholar]
- Ashburner, M. , Ball, C. A. , Blake, J. A. , Botstein, D. , Butler, H. , Cherry, J. M. , … Eppig, J. T. (2000). Gene ontology: Tool for the unification of biology. Nature Genetics, 25(1), 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz, S.‐G.‐G. , Fardyazar, Z. , Pashaei‐Asl, F. , Rahmati‐Yamchi, M. , Khodadadi, K. , & Pashaiasl, M. (2019). Human amniotic fluid stem cells (hAFSCs) expressing p21 and cyclin D1 genes retain excellent viability after freezing with (dimethyl sulfoxide) DMSO. Bosnian Journal of Basic Medical Sciences, 19(1), 43 10.1371/journal.pone.0158281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz, S.‐G.‐G. , Pashaei‐Asl, F. , Fardyazar, Z. , & Pashaiasl, M. (2016). Isolation, characterization, cryopreservation of human amniotic stem cells and differentiation to osteogenic and adipogenic cells. PLoS ONE, 11(7), e0158281 10.1371/journal.pone.0158281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei, G. , Aziz, Gholizadeh Ghaleh , , Alipour, S., , & Naderi, R. (2018). Comparison of traditional prenatal diagnosis procedures and Cell-Free DNA in maternal plasma as a new molecular approach for prenatal diagnosis. International Journal of Research in Applied and Basic Medical Sciences, 4(2), 108–118. [Google Scholar]
- Baker, S. J. , Markowitz, S. , Fearon, E. R. , Willson, J. , & Vogelstein, B. (1990). Suppression of human colorectal carcinoma cell growth by wild‐type p53. Science, 249(4971), 912–915. 10.1126/science.2144057 [DOI] [PubMed] [Google Scholar]
- Barbieri, F. , Lorenzi, P. , Ragni, N. , Schettini, G. , Bruzzo, C. , Pedulla, F. , & Alama, A. (2004). Overexpression of cyclin D1 is associated with poor survival in epithelial ovarian cancer. Oncology, 66(4), 310–315. 10.1159/000078332 [DOI] [PubMed] [Google Scholar]
- Bast, R. C. Jr , Hennessy, B. , & Mills, G. B. (2009). The biology of ovarian cancer: New opportunities for translation. Nature Reviews Cancer, 9(6), 415–428. 10.1038/nrc2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S. S. , & Koh, C. J. (2013). Bladder cancer cell in co‐culture induces human stem cell differentiation to urothelial cells through paracrine FGF10 signaling. Vitro Cellular & Developmental Biology‐Animal, 49(10), 746–751. 10.1007/s11626-013-9662-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin, J. C. , & Spinella, M. J. (2005). p53 in human embryonal carcinoma: Identification of a transferable, transcriptional repression domain in the N‐terminal region of p53. Oncogene, 24(9), 1481–1490. 10.1038/sj.onc.1208130 [DOI] [PubMed] [Google Scholar]
- El‐Deiry, W. S. , Tokino, T. , Velculescu, V. E. , Levy, D. B. , Parsons, R. , Trent, J. M. , … Vogelstein, B. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell, 75(4), 817–825. 10.1016/0092-8674(93)90500-p [DOI] [PubMed] [Google Scholar]
- Gholizadeh-Ghalehaziz, S. , Farahzadi, R. , Fathi, E. , & Pashaiasl, M. (2015). A mini overview of isolation, characterization and application of amniotic fluid stem cells. International journal of stem cells, 8(2), 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh-Ghaleh Aziz, S. , Fathi, E. , Rahmati-Yamchi, M. , Akbarzadeh, A. , Fardyazar, Z. , & Pashaiasl, M. (2017). An update clinical application of amniotic fluid-derived stem cells (AFSCs) in cancer cell therapy and tissue engineering. Artificial cells, nanomedicine, and biotechnology, 45(4), 765–774. [DOI] [PubMed] [Google Scholar]
- Harper, J. W. , Adami, G. R. , Wei, N. , Keyomarsi, K. , & Elledge, S. J. (1993). The p21 Cdk‐interacting protein Cip1 is a potent inhibitor of G1 cyclin‐dependent kinases. Cell, 75(4), 805–816. 10.1016/0092-8674(93)90499-g [DOI] [PubMed] [Google Scholar]
- He, Y.‐F. , Ji, C.‐S. , Hu, B. , Fan, P.‐S. , Hu, C.‐L. , Jiang, F.‐S. , … Wang, W. (2013). A phase Ⅱ study of paclitaxel and nedaplatin as front‐line chemotherapy in Chinese patients with metastatic esophageal squamous cell carcinoma. World Journal of Gastroenterology, 19(35), 5910–5916. 10.3748/wjg.v19.i35.5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocente, S. A. , Abrahamson, J. L. , Cogswell, J. P. , & Lee, J. M. (1999). p53 regulates a G2 checkpoint through cyclin B1. Proceedings of the National Academy of Sciences, 96(5), 2147–2152. 10.1073/pnas.96.5.2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackstadt, R. , Jung, P. , & Hermeking, H. (2013). AP4 directly downregulates p16 and p21 to suppress senescence and mediate transformation. Cell Death & Disease, 4(8), e775 10.1038/cddis.2013.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , Sheikh, M. S. , & Huang, Y. (2010). Decision making by p53: Life versus death. Molecular and Cellular Pharmacology, 2(2), 69. [PMC free article] [PubMed] [Google Scholar]
- Jiang, W. , Kahn, S. M. , Tomita, N. , Zhang, Y. J. , Lu, S. H. , & Weinstein, I. B. (1992). Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Research, 52(10), 2980–2983. [PubMed] [Google Scholar]
- Jiao, H. , Guan, F. , Yang, B. , Li, J. , Song, L. , Hu, X. , & Du, Y. (2012). Human amniotic membrane derived‐mesenchymal stem cells induce C6 glioma apoptosis in vivo through the Bcl‐2/caspase pathways. Molecular Biology Reports, 39(1), 467–473. 10.1007/s11033-011-0760-z [DOI] [PubMed] [Google Scholar]
- Kang, N. , Hwang, K. , Kim, S. , Kim, Y. , Hyun, S. , Jeung, E. , & Choi, K. (2012). Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid‐derived stem cells. Cancer Gene Therapy, 19(8), 517–522. 10.1038/cgt.2012.30 [DOI] [PubMed] [Google Scholar]
- Kang, N. , Hwang, K. , Yi, B. , Lee, H. , Jeung, E. , Kim, S. , & Choi, K. (2012). Human amniotic fluid‐derived stem cells expressing cytosine deaminase and thymidine kinase inhibits the growth of breast cancer cells in cellular and xenograft mouse models. Cancer Gene Therapy, 19(6), 412–419. 10.1038/cgt.2012.15 [DOI] [PubMed] [Google Scholar]
- Kang, N.‐H. , Yi, B.‐R. , Lim, S. Y. , Hwang, K.‐A. , Baek, Y. S. , Kang, K.‐S. , & Choi, K.‐C. (2012). Human amniotic membrane‐derived epithelial stem cells display anticancer activity in BALB/c female nude mice bearing disseminated breast cancer xenografts. International Journal of Oncology, 40(6), 2022–2028. [DOI] [PubMed] [Google Scholar]
- Kim, A. , Ueda, Y. , Naka, T. , & Enomoto, T. (2012). Therapeutic strategies in epithelial ovarian cancer. Journal of Experimental and Clinical Cancer Research, 31(1), 14 10.5772/39132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Lee, Y. , Kim, H. , Hwang, K. , Kwon, H. , Kim, S. , … You, J. (2007). Human amniotic fluid‐derived stem cells have characteristics of multipotent stem cells. Cell Proliferation, 40(1), 75–90. 10.1111/j.1365-2184.2007.00414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, P. , & Crawford, L. (1986). Characterization of the human p53 gene. Molecular and Cellular Biology, 6(5), 1379–1385. 10.1128/mcb.6.5.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen, C. N. Jr , Birrer, M. J. , & Sood, A. K. (2008). Early events in the pathogenesis of epithelial ovarian cancer. Journal of Clinical Oncology, 26(6), 995–1005. 10.1200/jco.2006.07.9970 [DOI] [PubMed] [Google Scholar]
- Li, L. , Wang, D. , Zhou, J. , Cheng, Y. , Liang, T. , & Zhang, G. (2015). Characteristics of human amniotic fluid mesenchymal stem cells and their tropism to human ovarian cancer. PLoS ONE, 10(4), e0123350 10.1371/journal.pone.0123350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lynch, H. T. , Casey, M. J. , Snyder, C. L. , Bewtra, C. , Lynch, J. F. , Butts, M. , & Godwin, A. K. (2009). Hereditary ovarian carcinoma: Heterogeneity, molecular genetics, pathology, and management. Molecular Oncology, 3(2), 97–137. 10.1016/j.molonc.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magatti, M. , De Munari, S. , Vertua, E. , & Parolini, O. (2012). Amniotic membrane‐derived cells inhibit proliferation of cancer cell lines by inducing cell cycle arrest. Journal of Cellular and Molecular Medicine, 16(9), 2208–2218. 10.1111/j.1582-4934.2012.01531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluggage, W. G. (2011). Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology‐Journal of the RCPA, 43(5), 420–432. 10.1097/pat.0b013e328348a6e7 [DOI] [PubMed] [Google Scholar]
- Network, C. G. A. R. (2011). Integrated genomic analyses of ovarian carcinoma. Nature, 474(7353), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknejad, H. , Khayat‐Khoei, M. , Peirovi, H. , & Abolghasemi, H. (2014). Human amniotic epithelial cells induce apoptosis of cancer cells: A new anti‐tumor therapeutic strategy. Cytotherapy, 16(1), 33–40. 10.1016/j.jcyt.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Norbury, C. , & Nurse, P. (1992). Animal cell cycles and their control. Annual Review of Biochemistry, 61(1), 441–468. 10.1146/annurev.biochem.61.1.441 [DOI] [PubMed] [Google Scholar]
- Pashaei‐Asl, F. , Pashaei‐Asl, R. , Khodadadi, K. , Akbarzadeh, A. , Ebrahimie, E. , & Pashaiasl, M. (2018). Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artificial Cells, Nanomedicine, and Biotechnology, 46(7), 1483–1487. 10.1080/21691401.2017.1374281 [DOI] [PubMed] [Google Scholar]
- Perin, L. , Sedrakyan, S. , Da Sacco, S. , & De Filippo, R. (2008). Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods in Cell Biology, 86, 85–99. 10.1016/s0091-679x(08)00005-8 [DOI] [PubMed] [Google Scholar]
- Pines, J. , & Hunter, T. (1989). Isolation of a human cyclin cDNA: Evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34 cdc2. Cell, 58(5), 833–846. 10.1016/0092-8674(89)90936-7 [DOI] [PubMed] [Google Scholar]
- Rahbar, S. , Novin, M. , Alizadeh, E. , Shahnazi, V. , Pashaei‐Asl, F. , AsrBadr, Y. , … Pashaiasl, M. (2017). New insights into the expression profile of MicroRNA‐34c and P53 in infertile men spermatozoa and testicular tissue. Cellular and Molecular Biology (Noisy‐le‐Grand, France), 63(8), 77–83. 10.14715/cmb/2017.63.8.17 [DOI] [PubMed] [Google Scholar]
- Rodriguez, R. , & Meuth, M. (2006). Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Molecular Biology of the Cell, 17(1), 402–412. 10.1091/mbc.e05-07-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, I. , & Bast, R. C. Jr (2012). Minireview: Human ovarian cancer: Biology, current management, and paths to personalizing therapy. Endocrinology, 153(4), 1593–1602. 10.1210/en.2011-2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata‐Kobayashi, S. , Yamashita, H. , Okuma, K. , Shiraishi, K. , Igaki, H. , Ohtomo, K. , & Nakagawa, K. (2013). Correlation among 16 biological factors [p53, p21waf1, MIB‐1 (Ki‐67), p16INK4A, cyclin D1, E‐cadherin, Bcl‐2, TNF‐α, NF‐κB, TGF‐β, MMP‐7, COX‐2, EGFR, HER2/neu, ER, and HIF‐1α] and clinical outcomes following curative chemoradiation therapy in 10 patients with esophageal squamous cell carcinoma. Oncology Letters, 5(3), 903–910. 10.3892/ol.2013.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skomedal, H. , Kristensen, G. B. , Abeler, V. M. , Børresen‐dale, A. L. , Tropé, C. , & Holm, R. (1997). TP53 protein accumulation and gene mutation in relation to overexpression of MDM2 protein in ovarian borderline tumours and stage I carcinomas. The Journal of Pathology, 181(2), 158–165. [DOI] [PubMed] [Google Scholar]
- Stuckey, D. W. , & Shah, K. (2014). Stem cell‐based therapies for cancer treatment: Separating hope from hype. Nature Reviews Cancer, 14(10), 683–691. 10.1038/nrc3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, M. (2012). Apoptosis, angiogenesis and cancer therapies. Journal of Cancer Therapeutics and Research, 1(1), 3 10.7243/2049-7962-1-3 [DOI] [Google Scholar]
- Wang, L. , Wu, Q. , Qiu, P. , Mirza, A. , McGuirk, M. , Kirschmeier, P. , … Liu, S. (2001). Analyses of p53 target genes in the human genome by bioinformatic and microarray approaches. Journal of Biological Chemistry, 276(47), 43604–43610. 10.1074/jbc.m106570200 [DOI] [PubMed] [Google Scholar]
- Xiong, Y. , Hannon, G. J. , Zhang, H. , Casso, D. , Kobayashi, R. , & Beach, D. (1993). p21 is a universal inhibitor of cyclin kinases. Nature, 366(6456), 701–704. 10.1038/366701a0 [DOI] [PubMed] [Google Scholar]
- You, Q. , Yao, Y. , Zhang, Y. , Fu, S. , Du, M. , & Zhang, G. (2015). Effect of targeted ovarian cancer therapy using amniotic fluid mesenchymal stem cells transfected with enhanced green fluorescent protein‐human interleukin‐2 in vivo. Molecular Medicine Reports, 12(4), 4859–4866. 10.3892/mmr.2015.4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. , Hu, W. , Deavers, M. T. , Shen, D.‐Y. , Fu, S. , Li, Y.‐F. , & Kavanagh, J. J. (2009). Nuclear cyclin B1 is overexpressed in low‐malignant‐potential ovarian tumors but not in epithelial ovarian cancer. American Journal of Obstetrics and Gynecology, 201(4), 367.e1–367.e6. 10.1016/j.ajog.2009.05.021 [DOI] [PubMed] [Google Scholar]


