Abstract
Background
Fatty acid amide hydrolase (FAAH) is an enzyme that prominently degrades the major endocannabinoid N-arachidonoylethanolamine (anandamide; AEA). Inhibition of this enzyme leads to increased AEA levels in brain regions that modulate stress and anxiety. Recently, we found that genetically-selected Marchigian Sardinian alcohol-preferring (msP) rats display hyperactive FAAH in amygdalar regions that was associated with increased stress sensitivity and a hyper-anxious phenotype. Our previous work has also demonstrated that msPs display an innate preference for and excessive consumption of alcohol, potentially reflecting a form of self-medication to gain relief from hyper-anxious states.
Methods
Here, we expand on our previous work by microinjecting the selective FAAH inhibitor URB597 (vehicle, 0.03, 0.1 and 1.0 μg/rat) into the central (CeA) and basolateral (BLA) amygdala in msP versus non-selected Wistar rats to evaluate the effects of localized FAAH inhibition on operant alcohol self-administration and restraint-induced anxiety using the elevated plus maze.
Results
Intra-CeA URB597 significantly reduced alcohol self-administration in msP, but not Wistar rats. Intra-BLA URB597 also attenuated alcohol drinking in msPs, although the effect was less pronounced relative to CeA treatment. In contrast, control experiments administering URB597 into the ventral tegmental area produced no genotypic differences in drinking. We also found that URB597 treatment in the CeA significantly reduced the anxiogenic effects of restraint stress in msPs, although no effects were detected in Wistars.
Conclusions
Dysregulation of FAAH regulated systems in the major output region of the amygdala may drive the propensity for comorbid expression of anxiety and excessive alcohol use.
Keywords: Addiction, alcoholism, abuse, ethanol, stress
INTRODUCTION
Alcohol abuse is often co-diagnosed with major psychiatric illnesses that may contribute to the development of alcohol dependence. For instance, alcohol use disorders (AUD) are comorbid with pathological anxiety in humans (Goodwin and Stein, 2004; Kushner et al., 2000; Smith and Randall, 2012), and have led to the “self-medication hypothesis”, in which alcohol drinking may serve to alleviate distress caused by negative affective states (Kushner et al., 2000). Under these circumstances, alcohol consumption is progressively escalated and AUDs develop more perniciously relative to circumstances void of preexisting mood disorders. Amygdaloid structures, particularly in the central nucleus division (CeA), may represent a major integrative hub for the intersection of stress signaling systems and cortico-limbic processes that augment the expression of comorbidity. (Gilpin et al., 2015; Koob, 2008, 2009). In this regard, the amygdala plays a critical role in driving alcohol intake to offset the negative affective symptoms of withdrawal in alcohol-dependent individuals (Kushner et al., 2005; Lovinger, 2012; Wang et al., 2003). In addition, evidence of the direct role of this area in the regulation of alcohol drinking has emerged in earlier preclinical work (Hyytia and Koob, 1995).
Genetically-selected Marchigian Sardinian alcohol-preferring (msP) rats display comorbid symptoms of anxiety and excessive alcohol-drinking. Our prior work has shown that a major contributor of elevated intake in this rodent model relates to the attenuation of anxiety- and depressive-like behaviors, consistent with the predictions of the “self-medication” model in alcohol-dependent individuals (Ciccocioppo et al., 2006; Ciccocioppo et al., 1999; Hansson et al., 2007; Hansson et al., 2006). Of importance, we have shown that msPs display pronounced aberrations in brain stress signaling that are commensurate with anxiety-like predispositions. Notably, msPs display an upregulation of corticotropin-releasing factor 1 (CRF1) receptor systems in the amygdala linked to a single nucleotide polymorphism in the promoter region of the gene (Ayanwuyi et al., 2013; Gray et al., 2015; Hansson et al., 2007; Hansson et al., 2006). MsPs also display evidence of dysregulated anti-stress systems in the CeA, as in the example of the neuropeptide nociceptin that normally suppresses the effects of stress and anxiety (Ciccocioppo et al., 2014). More recently, our studies involving the endogenous cannabinoid (eCB) system offer further insight into the role of dysfunctional anti-stress mechanisms in the CeA of msP rats (Natividad et al., 2017). The discovery of dysregulated eCB signaling in msPs raises the possibility that the eCB system may critically underlie the comorbid expression of behavioral anxiety and excessive alcohol drinking (Giuffrida et al., 2001; Huggins et al., 2012).
The major eCB, N-arachidonoylethanolamine (anandamide; AEA), is synthetized on-demand in post-synaptic neurons and retroactively acts on cannabinoid type 1 (CB1) receptors in pre-synaptic terminals to suppress neurotransmitter release (Diana and Marty, 2004; Marsicano and Lutz, 1999; Marsicano et al., 2002). In response to stressors, there is a rapid induction of AEA clearance mediated by the serine hydrolase fatty acid amide hydrolase (FAAH) in the amygdala, thus counteracting the anxiolytic properties of AEA (Gorzalka and Hill, 2009; Hill et al., 2009). Recent observations demonstrate that the loss of AEA-CB1 receptor signaling in the basolateral amygdala (BLA) is critical in modulating the behavioral and neuroendocrine responses to stress (Newsom et al., 2012; Ramikie and Patel, 2012). For example, the removal of AEA-mediated inhibitory control is shown to increase BLA excitability (Patel et al., 2005), activate the hypothalamic-pituitary-adrenal (HPA) axis and facilitate anxiety-like behavior (Ganon-Elazar and Akirav, 2009; Hill et al., 2009). In agreement with these findings, intra-BLA treatment with a FAAH inhibitor produces anxiolytic effects that are blocked with a CB1 receptor antagonist (Gunduz-Cinar et al., 2013b; Hill et al., 2009; Hill and Patel, 2013). Using msP rats, these mechanisms were recently extended into the CeA region, where an innate hyperfunction of the CRF1 receptor system was linked to upregulated amygdalar FAAH activity, resulting in diminished basal AEA tone and hyperexcitability of stress-reactive circuits in the CeA (Natividad et al., 2017).
Taken together, these studies suggest the possibility that innate increases in AEA degradation in the CeA may critically underlie the comorbid expression of anxiety-like behavior and alcohol drinking in msPs. Based on this hypothesis, we predict that selective FAAH inhibition in the amygdala would likely reduce symptoms of comorbidity in msP rats. To test this hypothesis, we delivered the selective FAAH inhibitor URB597 into the CeA and BLA regions, and evaluated subsequent responses on operant alcohol self-administration and stress-induced anxiety-like behavior. Control experiments were carried out by administering URB597 into the ventral tegmental area (VTA), a midbrain region where we might not expect to observe genotypic differences in comorbidity.
MATERIALS AND METHODS
Animals
The studies were conducted in male Wistar (Charles River, Calco, Italy) and msP rats bred at the School of Pharmacy (University of Camerino). Rats weighed approximately 200–225 g at the beginning of each study, and were housed in common cages contained within a temperature- and humidity-controlled vivarium (20–22°C; 45–55%) under a reverse 12:12h light/dark cycle (lights off at 9:00 a.m.). During the experiments, animals were given ad-libitum access to tap water and food pellets (4RF18, Mucedola, Settimo Milanese, Italy). All experimental sessions were conducted during the rats’ dark phase. Animals were treated in accordance to the guidelines of the European Community Council Directive for Care and Use of Laboratory Animals.
Drug Administration
The selective FAAH inhibitor, URB597 was purchased from Sigma SRL (Milano,Italy), and dissolved in 20% (v/v) solution of DMSO diluted in sterile isotonic saline.
Intracranial surgery
Animals were anesthetized by intramuscular injection (100–150 μl) of a solution containing tiletaminechlorohydrate (58.17 mg/ml) and zolazepamchlorohydrate (7.5 mg/ml). For drug injections, guide cannulas (0.65 mm outside diameter) were stereotaxically implanted bilaterally and cemented onto the skull. We used the following stereotaxic coordinates relative to bregma (in mm): CeA, anteroposterior (AP)-1.6, lateral (L) ± 5.0, and ventral (V) 7.0; BLA, (AP)-1.4, lateral (L) ± 5.8, and ventral (V) 8.0; VTA, (AP), −6,0; lateral (L), ± 2,2; ventral (V), −7,4; angle 12°. All coordinates were based on the Paxinos and Watson atlas (1998) and were adjusted for the body weight of the animals. Surgery was followed by a 7-day recovery period, during which time rats were left undisturbed in their home cages.
At the completion of the experiments, we injected 0.5μl/site of black India ink into the CeA, BLA or VTA. Rats were then immediately euthanized and histologically examined for cannula placement. All other drug or vehicle solutions were injected bilaterally at a volume of 0.3μl/site through a stainless-steel injector that was 1.5 mm longer than the guide cannula, allowing for the tip of the injector to protrude into the area under investigation.
Acute Restraint Stress
To elicit acute stress, msP and Wistar rats were restrained for 1 h in a cylindrical tube made of clear Plexiglas® and measuring 21.5 cm long with an internal diameter of 6.3 cm. The sliding plugs allowed for adjustments in length based on the animals’ size.
Rats from both genotypes were divided in two groups (restrained and non-restrained animals). The resulting four groups were then divided into subgroups (N = 6 – 7 group) receiving intracranial injections of URB597 (1.0 μg/rat) or vehicle (0.0 μg/rat). Solutions were administered 15 min prior to the incidence of restraint procedures. After 1 h restraint, rats were returned to their home cages, where they remained for 15 minutes before undergoing evaluation of anxiety-like behavior in the elevated plus maze test.
Elevated Plus Maze (EPM)
The EPM apparatus was located in a sound-attenuated room illuminated by red light (30 lux). It consisted of two black wooden open arms and two enclosed arms (40 cm high walls), arranged so that the similar arms were opposite of each other. Each 5-min trial began when the animal was placed in the center of the maze, facing a closed arm. A rat was considered to be on the central platform when at least two of its paws were placed inside these dimensions. An entry was defined as the presence of all four paws inside the respective arm. The number of open- and closed-arm entries, as well as the time spent in each arm were recorded. The percentage of time spent in the open arms [% OAT = (time in open arm/time in “open arm” + time in “closed” arm) × 100] and the percentage of open arm entries [% OAE = (number of open arm entries/number of “open + closed” arm entries) × 100] are considered to be a reliable index of anxiety-like behavior, whereas the number of total arm entries was used as an index of generalized locomotor activity (Pellow et al., 1986; Cippitelli et al.,2011; Domi et al., 2016).
Operant Alcohol Self-Administration
Operant training and testing were performed in self-administration chambers (Med Associates) equipped with a drinking reservoir (volume capacity 0.30 ml) and two retractable levers. Visual stimuli were presented via a light located on the front panel. Rats were trained to self-administer 10% alcohol (v/v) in 30-min daily sessions on a fixed-ratio 1 (FR-1) schedule of reinforcement. To facilitate self-administration training, we adapted a saccharin-fading procedure from Weiss et al., (1990). Briefly, during the first 5 days of training, active lever presses were reinforced by the delivery of 0.2 % (w/v) saccharin solution into the drinking receptacle. After the acquisition of the saccharin-reinforced response, alcohol was added to the solution and progressively increased to the final concentration of 10% (v/v) ethanol. Saccharin was progressively faded out. The delivery of the solutions was followed by a 5-s time-out period during which time the reinforced lever remained inactive. Each response resulted in the delivery of 0.1 ml of fluid. The number of operant responses of both the active and inactive levers, as well as the number of reinforcers received were recorded.
Statistical Analysis
Data were analyzed using within-or between-factor analyses of variance (ANOVA), or within-group paired t-tests. ANOVAs were followed by Newman-Keuls post-hoc tests where appropriate. Statistical significance was set at p<0.05. Animals with incorrect cannula placements were excluded from further analysis (n= 3 for the VTA, n=4 for the CeA and n=1 for the BLA).
RESULTS
Experiment 1: Effect of URB597 microinjection into the CeA on alcohol self-administration in msP and Wistar rats
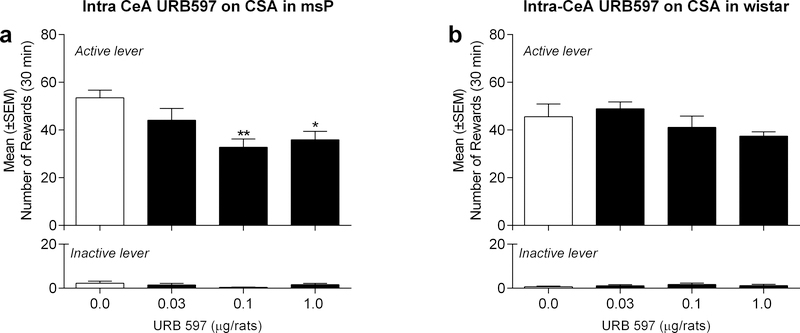
We administered URB597 or vehicle treatment following the acquisition of a stable baseline of alcohol self-administration. Treatments were administered 5 min before the beginning of the self-administration session, with rats receiving intra-CeA injections of URB597 (0.03, 0.1 and 1.0 μg/rat) or vehicle in a counterbalanced manner. Drug treatment was performed every fourth day of testing. The day after drug testing, rats remained in their home cages, whereas for the following two days, baseline alcohol self-administration was reestablished prior to the subsequent drug test. A one-way, within-subject ANOVA in msP rats (n=10) revealed a main effect of treatment [F(3,27)=5.41, p<0.01]. As shown in Figure 1a, Newman-Keuls post-hoc tests confirmed a significant difference between vehicle controls and rats that were administered URB597 at the 0.1 (p<0.01) and 1.0 μg/rat doses (p<0.05). Specifically, URB597 treatment markedly reduced operant responses on the alcohol-reinforced lever (Fig 1a), whereas responses on the inactive lever remained negligible and unaltered by URB597 treatment [F(3,27)=2.06, p=ns]. Similar analyses in Wistar rats (n=9) revealed no overall effect of URB597 treatment on alcohol self-administration [F(3,26)=1.8, p=ns], or inactive lever responding [F(3,26)=0.7, p=ns] (Fig. 1b).
Figure 1:
Effect of intra-CeA injections of URB597 on alcohol self-administration in a) msP rats and b) Wistar rats. URB597 at the doses of 0.1 and 1.0 μg/rat decreased alcohol-reinforced active lever responding in msP but not Wistar rats. Values represent the mean ± (SEM) of number of rewards obtained by active lever responding (top panel) and responses on the inactive lever (bottom panel). Significant differences from vehicle-treated rats (Veh): *p<0.05, **p<0.01.
Experiment 2: Effect of URB597 microinjection into the BLA on alcohol self-administration in msP and Wistar rats
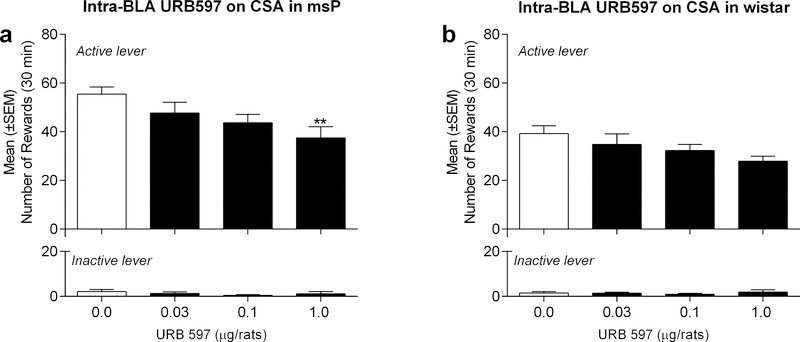
We followed similar treatment strategies as mentioned above in separate cohorts of msP rats (n=7) receiving intra-BLA injections of URB597 (0.03, 0.1 and 1.0 μg/rat) or vehicle. A one-way, within-subject ANOVA revealed an overall effect of treatment [F(3,6)=5.0, p<0.05]. As shown in Figure 2a, Newman-Keuls post-hoc tests confirmed the efficacy of URB597 treatment at the highest dose (1.0 μg/rat) that reduced alcohol self-administration as compared to vehicle controls (Fig. 2a). Responding on the inactive lever was once again negligible and unaltered by drug treatment [F(3,6)= 0.91, p=ns]. Similar analyses in Wistar rats (n=10) revealed no overall effect of URB597 treatment on alcohol self-administration [F(3,9)=2.5, p=ns], or inactive lever responding [F(3,9)=0.34, p=ns] (Fig. 2b).
Figure 2:
Effect of intra-BLA injections of URB597 on alcohol self-administration in a) msP rats and b) Wistar rats. URB597 at the dose of 1.0 μg/rat decreased alcohol-reinforced responding in msP but not Wistar rats. Values represent the mean ± (SEM) of number of rewards obtained by active lever responding (top panels) and responses on the inactive lever (bottom panels). Significant differences from vehicle-treated rats (Veh), **p<0.01
Experiment 3: Effect of URB597 microinjection into the VTA on alcohol self-administration in msP rats
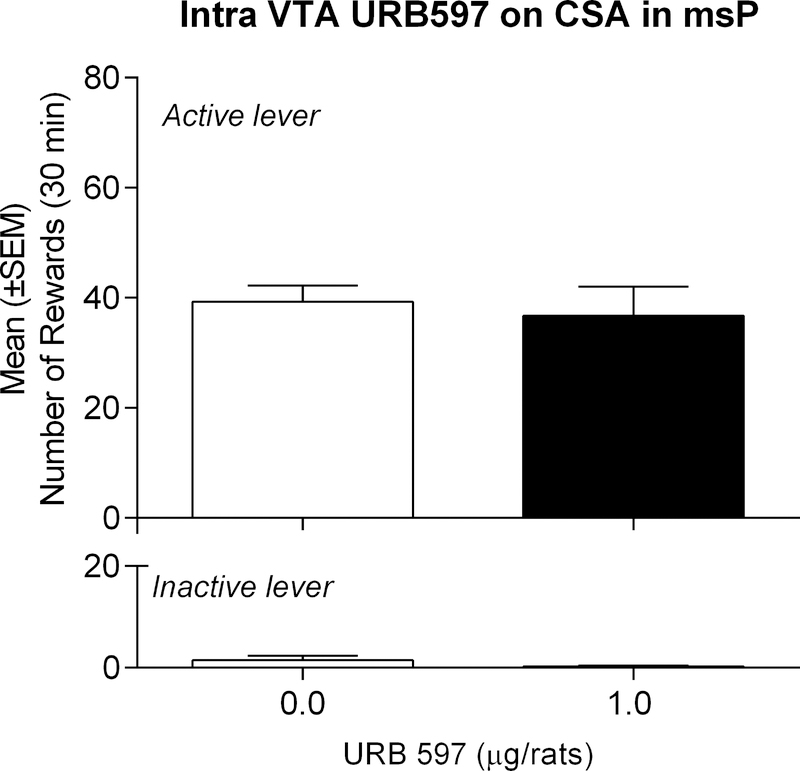
Control experiments were conducted on an additional group of msP rats (n=9) receiving intra-VTA injections of URB597 (1.0 μg/rat) or vehicle. We followed similar strategies as mentioned above in msPs only, given that intra-amygdalar URB597 treatment had no behavioral consequences in Wistars. Moreover, we evaluated operant responding at the dose that produced maximal effects in msPs. A one-way, within-subject ANOVA revealed no overall effect of URB597 treatment on alcohol self-administration [t(8)=0.4967, p=ns], or inactive lever responding [t(8)=1.244, p=ns] (Fig. 3).
Figure 3:
Effect of intra-VTA injections of URB597 on alcohol self-administration in msP rats. URB597 did not alter alcohol-reinforced active lever responding. Values represent the mean ± (SEM) of number of rewards obtained by active lever responding and responses on the inactive lever.
Experiment 4: Effect of URB597 microinjection into the CeA on restraint-induced anxiety-like behavior in msP and Wistar rats
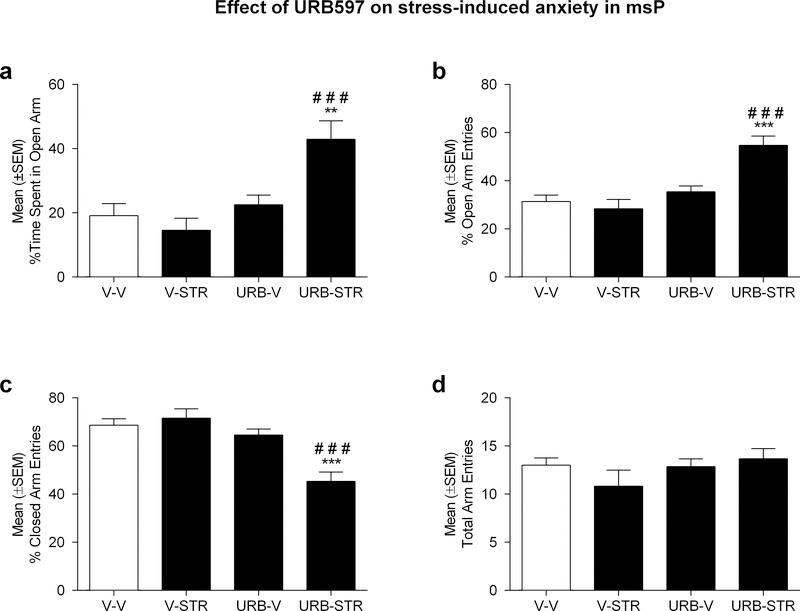
A two-way, between-subject ANOVA of the percentage of time spent in the open arms in msP rats (n=6/7 group) revealed a main effect of intra-CeA URB597 treatment [F(1,22)=14.99; p<0.001], as well as a treatment by stress interaction [F(1,22)=9.28; p<0.01]. As shown in Figure 4a, Newman-Keuls post-hoc tests revealed that URB597 treatment (1.0 μg/rat) significantly increased the percentage of time spent in the open arms of the EPM in restrained rats as compared to vehicle controls (p<0.001). The anxiolytic effect produced by URB597 treatment was observed only in animals that were subjected to restraint procedures (p<0.01).
Figure 4:
Effect of intra-CeA injections of URB597 (1.0 μg/rat) in msP rats on: a) % time spent in open arms; b) % number of open arm entries, c) % number of closed arm entries, d) total arm entries. URB597 exerted an anxiolytic-like effect in rats subjected to restraint stress as indicated by a higher percentage of time spent in the open arms. The effect of URB597 on open and closed arm entries are consistent with evidence of increased time spent exploring the open arms. Data are represented as the mean ± SEM. ###p<0.001 statistical differences versus V-STR (vehicle, restrained animals); **p<0.01***p<0.001 statistical differences versus V-V (vehicle, non-restrained animals).
Similar analyses of the percentage of open arm entries revealed an overall effect of treatment [F(1,22)=22.93; p<0.001], stress [F(1,22)=6.65; p<0.05], and treatment by stress interaction [F(1,22)=12.29; p<0.01]. As shown in Figure 4b, Newman-Keuls post-hoc tests revealed that URB597 treatment (1.0 μg/rat) significantly increased the percentage of open arms entries in restrained rats as compared to vehicle controls (p<0.001). Consistent with our analyses of open arm time, URB597 treatment was effective only in animals that were subjected to restraint procedures (p<0.001).
Similar analyses of the percentage of closed arm entries revealed an overall effect of treatment [F(1,22)=22,87; p<0.001], stress [F(1,22)=6,64; p<0.05], and treatment by stress interaction [F(1,22)=12.28; p<0.01]. As shown in Figure 4c, Newman-Keuls post-hoc tests revealed that URB597 treatment (1.0 μg/rat), significantly reduced the percentage of closed arms entries (p<0.001) compared to animals that received vehicle. Consistent with our previous analyses, URB597 treatment was effective only in animals that were subjected to restraint procedures.
Finally, our analyses of the number of total arm entries revealed no main effects of drug treatment [F(1,22)=1.54; p=ns], stress [F(1,22)=0.39; p=ns], or interaction of these variables [F(1,22)=1.89; p=ns] (Fig. 4d).
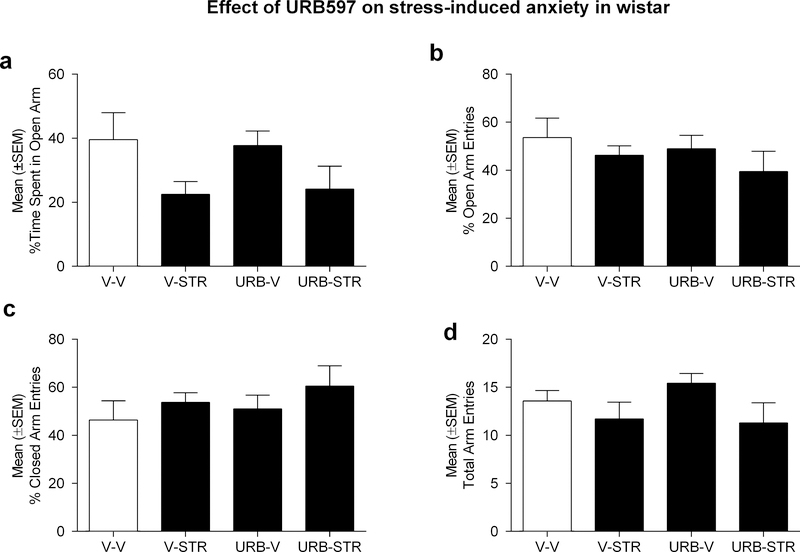
A two-way, between-subject ANOVA of the percentage of time spent in the open arms in Wistar rats (n=7group) revealed an overall effect of stress [F(1,24)= 5.9; p<0.05], although there was no main effect of treatment [F(1,24)= 0.0002; p=ns] or interaction of these variables [F(1,24)= 0.08; p=ns]. As shown in Figure 5a, restraint procedures induced a significant decrease in the percentage of time spent in the open arms, but did not appear to be influenced by URB597 treatment.
Figure 5:
Effect of intra-CeA injections of URB597 (1.0 μg/rat) in Wistar rats on a) % time spent in open arms; b) % number of open arm entries, c) % number of closed arm entries and d) total arm entries. As opposed to the data collected from msP rats, URB597 did not differentially alter EPM behaviors in Wistars. Data are represented as the mean ± SEM.
Similar analyses of the percentage of open arm entries revealed no main effects of drug treatment [F(1,24)=0.7; p=ns], stress[F(1,24)=1.5; p=ns] or interaction of these variables [F(1,24)=0.02; p=ns] (Fig. 5b). We also observed null findings for subsequent analyses of the percentage of closed arms entries [treatment: F(1,24)=0.7; p=ns, stress: F(1,24)=1.6; p=ns, treatment by stress: F(1,24)=0.02; p=ns, (Figure 5c)] and total arm entries [treatment: F(1,24)=0.2; p=ns, stress: F(1,24)=3.7; p=ns, treatment by stress: F(1,24)=0.5; p=ns, (Figure 5d)]
DISCUSSION
In summary, our studies revealed that blockade of FAAH-mediated hydrolysis in the amygdala significantly reduces comorbid expression of pathological anxiety and excessive alcohol drinking. Specifically, we report that intra-CeA, more so than intra-BLA URB597 treatment, attenuated operant alcohol self-administration in msPs showing innate preference for and spontaneous intake of alcohol. Additional work revealed that intra-CeA FAAH inhibition alleviated stress-induced anxiety-like behavior in msPs, but not Wistars. The selective response in the CeA extends our previous work examining the role of anti-stress mechanisms in modulating amygdalar output, and delineates a critical role of eCB signaling in driving comorbid symptoms of behavioral anxiety and excessive alcohol intake. The findings complement our recent work showing that the msP anxious phenotype is related in part to dysfunctional AEA signaling elements in the CeA (Natividad et al., 2017). Specifically, increased amygdalar FAAH activity in msPs is accompanied by reductions in dialysate AEA levels in the CeA, and is constitutively linked to the overexpression of CRF1 receptor signaling in the amygdala. Depending on the presence of two SNPs located close in proximity to the promoter region encoding for this receptor (Ayanwuyi et al., 2013; Cippitelli et al., 2015; Hansson et al., 2007; Hansson et al., 2006). Here, we provide behavioral evidence of site-specific amelioration of comorbid behaviors, consistent with the restoration of dysfunctional AEA signaling in the CeA. Our findings are consistent with emerging evidence supporting the role of the eCB system in modulating stress and anxiety (Bluett et al., 2014; Gray et al., 2015; Gunduz-Cinar et al., 2013a; Hill et al., 2013; Morena et al., 2016), and extend this work to include elements of pathological anxiety known to facilitate alcohol consumption.
Intra-amygdalar URB597 attenuated alcohol self-administration, although we observed regional distinctions in CeA versus BLA treatment. Of note, URB597 reduced alcohol self-administration at lower doses in the CeA versus BLA. The finding suggests that the effect of URB597 on alcohol intake may be strongly influenced by AEA signaling in the CeA, whereas the smaller effect observed in the BLA may have resulted from drug diffusion, given the close proximity of these amygdalar regions. While the involvement of the BLA region cannot be fully excluded, we observed that intra-VTA treatment failed to alter alcohol intake. The finding is significant in ruling out the possibility that URB597 may have acted on distal midbrain regions via drug diffusion into the brain parenchyma.
In contrast to the present results, our previous work in msPs showed that peripheral injections of URB597 did not influence alcohol drinking (Cippitelli et al., 2008). In other work conducted in a different alcohol-preferring line (i.e., Alko, Alcohol rats), it was reported that AA rats display decreased FAAH mRNA transcripts and enzyme activity in the prefrontal cortex (PFC), leading to locally overactive eCB transmission. Moreover, intra-PFC URB597 treatment was observed to enhance alcohol self-administration in non-selected Wistars (Hansson et al., 2007). An attractive explanation to reconcile these findings is that eCB mechanisms in the PFC and CeA may play opposing roles in the regulation of alcohol drinking. In this regard, enhanced eCB transmission may facilitate or inhibit alcohol drinking, depending on the brain region that is primarily affected (i.e. the PFC or CeA, respectively). As a result of these site-specific differences, peripherally-injected URB597 would likely increase eCB tone in both regions, resulting in a null effect on alcohol drinking.
A broad set of studies has shown that administration of URB597 exerts anxiolytic effects and attenuates stress responses, presumably by bolstering AEA signaling (Bedse et al., 2014; Gaetani et al., 2003; Hill et al., 2010). Noteworthy, msP rats are sensitized to stressors, show excessive anxiety and alcohol intake, and are motivated to consume alcohol to ameliorate innate symptoms of negative affect (Ciccocioppo et al., 2006). Based on these findings, we predicted that the inhibitory effect of URB597 on alcohol self-administration, that is specific for msP rats, was dependent on the ability of the drug to attenuate anxiety following CeA injection. To test this hypothesis, we evaluated anxiety-like behavior following intra-CeA URB597 at the same dose that produced maximal effects on alcohol self-administration. As expected, FAAH inhibition reduced anxiety-like effects in msPs but not Wistars, and was specific to the alleviation of stress-induced anxiety via restraint. Given the unique propensity for pathological anxiety, it is not surprising that msPs exhibited a floor effect in the EPM study, wherein reductions in open-arm time were likely occluded by their innate anxious demeanor (Ciccocioppo et al., 2006). Interestingly, Wistars demonstrated an anxiogenic response to restraint stress that was unaltered by URB597 treatment.
Our findings also underscore the significance of CeA endocannabinoid signaling, particularly in the correction of aberrant stress reactivity. In this regard, our previous work demonstrated that msPs exhibit heightened spontaneous excitatory signaling and increased basal glutamatergic levels in the CeA that are sensitized to stressors like restraint immobilization. In contrast, CeA glutamatergic transmission was not differentially altered by restraint in Wistar rats (Natividad et al., 2017). These findings prompt the hypothesis that local enhancement of endocannabinoid levels in the CeA may reduce problematic behaviors influenced by neuroadaptations in stress-reactive circuits in the amygdala. This is consistent with our recent data showing that FAAH inhibition attenuates stress-induced increases in CeA glutamatergic transmission (Natividad et al., 2017), presumably by bolstering deficient AEA tone in msPs. Noteworthy, FAAH co-localizes with CB1 receptors that are primarily expressed on glutamatergic terminals in CeA and via retrograde negative feedback mechanisms, exert inhibitory control of glutamatergic signaling. (Egertova et al., 2003; Egertova et al., 1998).
In conclusion, our results demonstrate that selective FAAH inhibition alleviates comorbid symptoms of anxiety and excessive alcohol drinking, which we propose are driven in part by chronic dysregulation of stress signaling in the major output region of the amygdala. Moreover, innate dysregulation of CeA endocannabinoid signaling may exacerbate the underlying pathology of comorbidity. Although it is anticipated that the therapeutic benefits of FAAH inhibitors are due to the amplification of AEA-CB1 signaling, it is worth mentioning that FAAH contributes to the degradation of other N-acylethanolamines such as oleoylethanolamide and palmitoylethanolamide. These molecules have been shown to exert anxiolytic effects and even reduce alcohol drinking through activation of PPARα and PPARγ receptors (Bilbao et al., 2016; Domi et al., 2016; Stopponi et al., 2013; Stopponi et al., 2011). Future studies should focus on systematically exploring the significance of these pathways in mediating the therapeutic benefits of FAAH inhibitors.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism via the following mechanisms: R37-AA017447 (MR and RC), R01-AA015566 (MR), and a Research Supplement to Promote Diversity (LAN). This manuscript is dedicated to our departed friend, colleague, and mentor Dr. Loren (Larry) Parsons, whose seminal contributions in the study of endocannabinoid signaling, stress, and drug addiction will be a driving force in the field for future generations.
REFERENCES
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A (2013) Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Frontiers in psychiatry 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedse G, Colangeli R, Lavecchia AM, Romano A, Altieri F, Cifani C, Cassano T, Gaetani S (2014) Role of the basolateral amygdala in mediating the effects of the fatty acid amide hydrolase inhibitor URB597 on HPA axis response to stress. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 24:1511–1523. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Serrano A, Cippitelli A, Pavon FJ, Giuffrida A, Suarez J, Garcia-Marchena N, Baixeras E, Gomez de Heras R, Orio L, Alen F, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2016) Role of the satiety factor oleoylethanolamide in alcoholism. Addiction biology 21:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S (2014) Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Translational psychiatry 4:e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M (2014) Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience 34:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M (2006) Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol 11:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M (1999) Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology 144:151–157. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Ayanwuyi LO, Barbier E, Domi E, Lerma-Cabrera JM, Carvajal F, Scuppa G, Li H, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R (2015) Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology 232:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R (2008) Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology 198:449–460. [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A (2004) Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). British journal of pharmacology 142:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi E, Uhrig S, Soverchia L, Spanagel R, Hansson AC, Barbier E, Heilig M, Ciccocioppo R, Ubaldi M (2016) Genetic Deletion of Neuronal PPARgamma Enhances the Emotional Response to Acute Stress and Exacerbates Anxiety: An Effect Reversed by Rescue of Amygdala PPARgamma Function. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:12611–12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR (2003) Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience 119:481–496. [DOI] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR (1998) A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proceedings Biological sciences 265:2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani S, Cuomo V, Piomelli D (2003) Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends in molecular medicine 9:474–478. [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I (2009) Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci 29:11078–11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M (2015) The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D (2001) Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. The Journal of pharmacology and experimental therapeutics 298:7–14. [PubMed] [Google Scholar]
- Goodwin RD, Stein MB (2004) Association between childhood trauma and physical disorders among adults in the United States. Psychological medicine 34:509–520. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN (2009) Integration of endocannabinoid signaling into the neural network regulating stress-induced activation of the hypothalamic-pituitary-adrenal axis. Curr Top Behav Neurosci 1:289–306. [DOI] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, Hill MN (2015) Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A (2013a) Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends in pharmacological sciences 34:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie TS, Gorka AX, Alapafuja SO, Nikas SP, Makriyannis A, Poulton R, Patel S, Hariri AR, Caspi A, Moffitt TE, Kunos G, Holmes A (2013b) Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular psychiatry 18:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M (2007) Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addiction biology 12:30–34. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R (2006) Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proceedings of the National Academy of Sciences of the United States of America 103:15236–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, McEwen BS (2013) Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Molecular psychiatry 18:1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V (2010) Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences of the United States of America 107:9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB (2009) Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34:2733–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S (2013) Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biology of mood & anxiety disorders 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins JP, Smart TS, Langman S, Taylor L, Young T (2012) An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 153:1837–1846. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF (1995) GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. European journal of pharmacology 283:151–159. [DOI] [PubMed] [Google Scholar]
- Koob GF (2008) A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2009) Brain stress systems in the amygdala and addiction. Brain Res 1293:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C (2000) The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clinical psychology review 20:149–171. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S (2005) Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res 29:1432–1443. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (2012) Young investigators stress alcohol-induced neuroadaptations in extended amygdala. Alcohol 46:299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. The European journal of neuroscience 11:4213–4225. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534. [DOI] [PubMed] [Google Scholar]
- Morena M, Leitl KD, Vecchiarelli HA, Gray JM, Campolongo P, Hill MN (2016) Emotional arousal state influences the ability of amygdalar endocannabinoid signaling to modulate anxiety. Neuropharmacology 111:59–69. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C, Polis I, Ciccocioppo R, Roberto M, Parsons LH (2017) Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom RJ, Osterlund C, Masini CV, Day HE, Spencer RL, Campeau S (2012) Cannabinoid receptor type 1 antagonism significantly modulates basal and loud noise induced neural and hypothalamic-pituitary-adrenal axis responses in male Sprague-Dawley rats. Neuroscience 204:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ (2005) Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology 30:497–507. [DOI] [PubMed] [Google Scholar]
- Ramikie TS, Patel S (2012) Endocannabinoid signaling in the amygdala: anatomy, synaptic signaling, behavior, and adaptations to stress. Neuroscience 204:38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Randall CL (2012) Anxiety and alcohol use disorders: comorbidity and treatment considerations. Alcohol research : current reviews 34:414–431. [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, Ubaldi M, Heilig M, Demopulos G, Gaitanaris G, Ciccocioppo R (2013) Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcoholism, clinical and experimental research 37:1351–1360. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R (2011) Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biological psychiatry 69:642–649. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G (2003) Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A 100:1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]