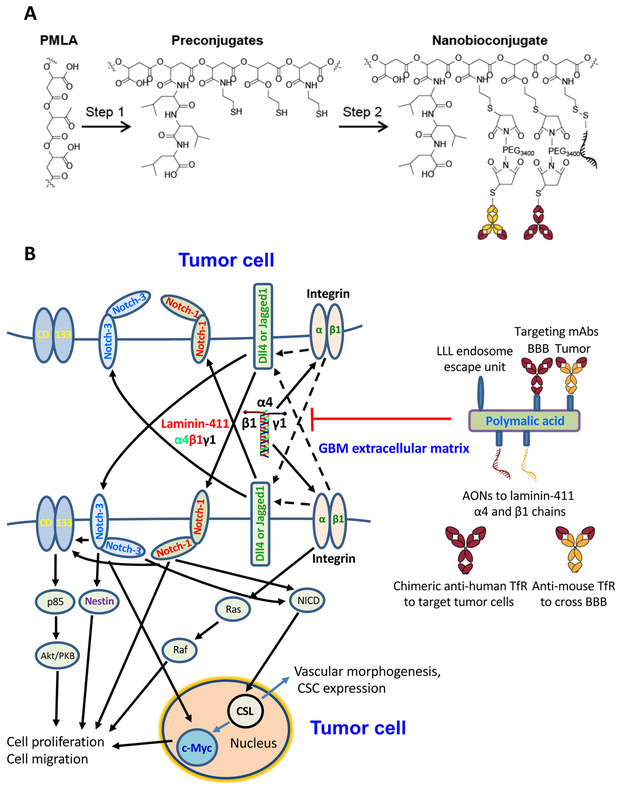
Fig. 6. Nanobioconjugates design, synthesis and schematic mechanism of drug action.
A. Nanodrug synthesis step 1: Poly(β-L-malic acid) (PMLA) was treated with a mixture of N-hydroxysuccinimide ester (NHS) and N,N′-dicyclohexylcarbodiimide (DCC) followed by sequential addition of trileucine (leucyl-N-leucyl-N-leucine [LLL]) peptide along with triethanolamine (TEA) and finally 2-mercapto-1-ethylamine (MEA) followed by TEA to form Preconjugate-1. Step 2: Monoclonal antibodies (mAbs) were functionalized with MAL-PEG-MAL and attached to the preconjugate by a thioether. Similarly, antisense oligonucleotides (AONs) were modified with a thiol reacting linker and attached to the preconjugate through a disulfide linkage. The cartoon schematic of the full nanobioconjugate is depicted at the lower left. Abbreviations: MAL, maleimide; PEG, polyethylene glycol; PEG3400, PEG with m.w.=3,400; BBB, blood-brain barrier; a-TfR, anti-transferrin receptor mAb. B. Schematic of Notch signaling and nanodrug action. Left, laminin-411 in the glioblastoma ECM interacts with β1 integrin on the tumor cell surface to upregulate Notch ligands, e.g., delta-like 4 (Dll4). This in turn activates Notch receptors by a juxtacrine or autocrine regulation documented in gliomas (31,32) (juxtacrine trans-signaling is shown). After Notch cleavage and release of Notch intracellular domain (NICD), the signal is transferred to the nucleus with activation of DNA-binding adaptor CSL/CBF-1, resulting in increased transcription and CSC proliferation and expansion. Interaction with and activation of additional CSC markers CD133, Nestin and c-Myc, leads to higher proliferation, migration and vascular morphogenesis. Right, therapeutic nanobioconjugate blocking laminin-411 chain expression. It has PMLA backbone with covalently attached components: morpholino AONs inhibiting laminin α4 and β1 chain synthesis; multiple residues of trileucine (LLL) functioning as a pH-sensitive endosome escape unit for the release of AON into the tumor cell cytoplasm; BBB and tumor targeting antibodies: anti-mouse TfR mAb targeting mouse endothelial cells in tumor vessels for the nanodrug BBB crossing by transcytosis, and chimeric anti-human TfR mAb enabling drug targeting and binding to human tumor cells with subsequent receptor-mediated internalization (see Supplementary Video).