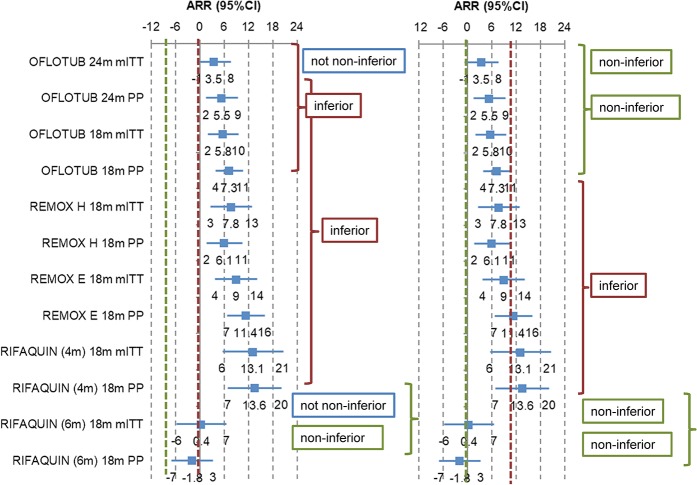
Fig 2. Unfavourable treatment outcome with fluoroquinolone-substitution regimens versus standard regimen for DSTB expressed as ARR with 95% CIs interpreted against a 6% (predetermined) and a 10% (post hoc) noninferiority margin.
18m, 18 month follow-up from treatment start; 24m, 24 month follow-up after treatment end; ARR, absolute risk reduction; CI, confidence interval; DSTB, drug-sensitive tuberculosis; mITT, modified intent-to-treat set; PP, per-protocol set.