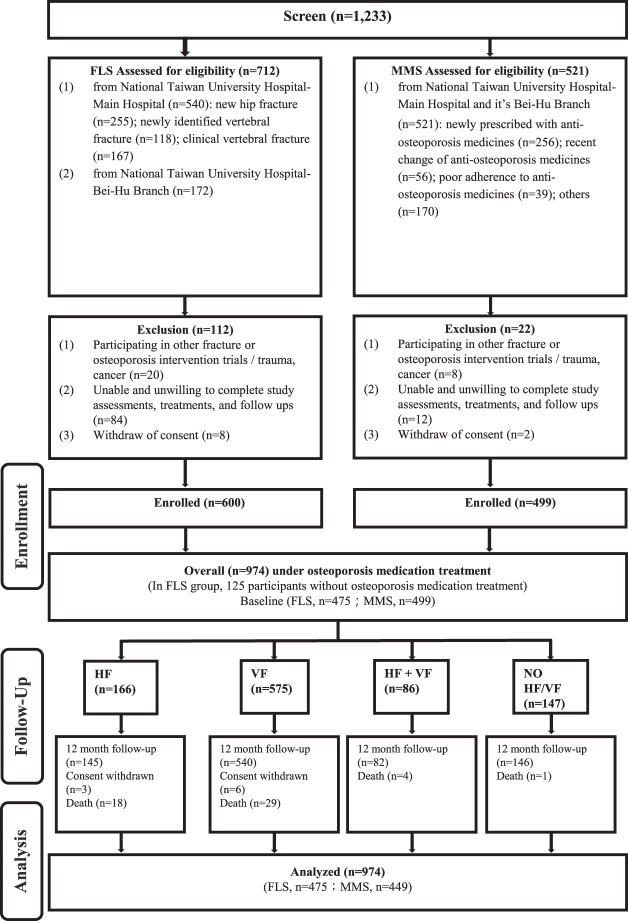
Figure 1.
Study flow chart. Participants were excluded from the service after screening if (1) the fractures were related to trauma, cancer, or atypical fracture at femoral shaft; (2) participating physicians felt that the patients’ life expectancy are ≦2 years; (3) unable and unwilling to complete study assessments and follow-up; (4) participating in other fracture or osteoporosis intervention trials. After enrollment, interventions were performed and they were followed for 12 months. Participants with osteoporosis medications (n = 475 in FLS group and n = 499 in MMS group) are regrouped into HF, VF, HF + VF and NO HF/VF for secondary data analysis. HF: participants with hip fracture, VF: participants with vertebral fracture. HF + VF: participants with hip fracture and vertebral fracture. NO HF/VF: participants with no hip fracture or vertebral fracture.