Abstract
Introduction
Macrophages play an important role in regulating inflammation and tissue regeneration. It is known that anti-inflammatory macrophages play an important role for tissue regeneration. The objective of this study is to modify macrophages phenotypes for anti-inflammatory function by utilizing drug delivery technology.
Method
In this study, 4 types of poly (L-lactic-co-glycolic acid) (PLGA) microspheres incorporating pioglitazone of an anti-inflammatory modifier (pio-MS) with different sizes were prepared. In vitro release test of pio-MS was performed in phosphate buffered-saline solution (PBS) containing 1 wt% of sodium lauryl sulfate. The arginase activity and the secretion of interleukin (IL)−10 as anti-inflammatory macrophage markers of mouse bone marrow derived-macrophages (BMDM) cultured with the pio-MS were evaluated.
Results
The sustained release of pioglitazone was observed from all types of pio-MS in vitro. When BMDM were cultured with the pio-MS with an average diameter of 40 μm (pio-MS40), the arginase activity and the secretion of IL-10 increased to a significant extent compared with other pio-MS.
Conclusions
The pio-MS40 with an diameter of 40 μm had a potential to induce the anti-inflammatory modification of BMDM in this culture system. The sustained release of pioglitazone is promoting to modify the macrophage function.
Keywords: Macrophages, Pioglitazone, Drug delivery system, Poly(L-lactic-co-glycolic acid), PLGA, Microspheres
Abbreviations: PLGA, poly(L-lactic-co-glycolic acid); pio-MS, PLGA microspheres incorporating pioglitazone; PBS, phosphate buffered-saline solution; IL, interleukin; BMDM, mouse bone marrow derived-macrophage; iNOS, inducible nitric oxide synthase; TNF, tumor necrosis factor; PPARγ, peroxisome proliferator-activated receptor γ; PLA, poly(l-lactic acid); PGA, poly(glycolic acid); DDW, double-distilled water; RS, resulting solution; SEM, scanning electron microscopy; HPLC, high performance liquid chromatography; UV, ultra violet; IMDM, Iscove's modified Dulbecco's medium; FBS, fetal bovine serum; M-CSF, macrophage colony stimulating factor; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; SD, standard deviation
Highlights
-
•
Microspheres incorporating pioglitazone with different sizes were prepared.
-
•
Sustained release of pioglitazone from the microspheres were observed.
-
•
The effect of pioglitazone on macrophages was enhanced by the sustained release.
1. Introduction
Macrophages have remarkable plasticity in physiology and change their phenotype in response to the environmental cues, resulting in the generation of various populations with different biological functions. It has been recognized that there are, at least, two phenotypes of macrophages, so-called M1 and M2. M1 macrophages with classically proinflammatory functions are typically present in inflammatory reactions and pathogen defense [1], [2], [3]. Generally, M1 macrophages produce interleukin (IL)-12, IL-23, inducible nitric oxide synthase (iNOS), toxic reactive oxygen, nitric oxygen intermediates, and inflammatory cytokines, such as IL-1β, IL-6, and tumor necrosis factor (TNF)-α. On the other hand, M2 macrophages with alternative non-inflammatory functions promote the responses of type 2 helper T cells associated with tumor progression [2], parasite infections, tissue repair [3], [4], and debris removal [4], [5], [6], [7]. M2 macrophages produce IL-10, high levels of scavenger, mannose, and galactose receptor, and arginase in the place of arginine [4], subsequently producing ornithine and polyamines.
The concepts and paradigm of macrophages polarization into M1 and M2 phenotypes have been noted in terms of inflammatory host responses to pathogens and cancer [3], [4], [8]. The macrophages phenotype also modifies the host response in disease pathogenesis, tissue injury, and the implantation of biomaterials [9]. The findings give us an idea that the inflammatory host responses may be modified by changing the macrophage phenotype.
Peroxisome proliferator-activated receptor γ (PPARγ) is one of the key factors to modify the M1/M2 phenotype ratio [10], [11], [12]. It is demonstrated that the activation of PPARγ potentiates the polarization of circulating monocytes to macrophages of M2 type and reduces the functions of M1 macrophages, attenuating macrophages-induced inflammatory reactions [13]. Among the PPARγ activators, pioglitazone is commercially available, and well-known as an agonist for PPARγ and a modulator of inflammatory responses [14]. The biological function has been demonstrated in in vitro cell culture systems. However, the biological function for macrophages phenotype is not always expected in vivo because of the in-stability. As one trial to tackle this issue, the local controlled release is promising by making use of drug delivery system (DDS) technology.
Poly (L-lactic-co-glycolic acid) (PLGA), a copolymer of poly (l-lactic acid) (PLA) and poly (glycolic acid) (PGA), has been intensively investigated for the delivery carrier of drugs [15], [16], [17], [18], [19], [20]. With the degradation, l-lactic acid and glycolic acid of final products are generated and both biologically metabolized by the surrounding cells through normal metabolic pathways [21]. The cytotoxicity has been clinically confirmed. The biocompatibility, low toxicity, and drug encapsulation capabilities of PLGA are suitable for the matrix materials of DDS [15], [16], [17], [18], [19], [20]. Based on this, several researches using PLGA have been reported on the DDS applications [16], [22], [23], [24], [25].
The objective of this study is to enhance the effect of pioglitazone to modify macrophages phenotype for M2 macrophages by utilizing drug delivery technology. In this study, PLGA microspheres incorporating pioglitazone (pio-MS) with different sizes were prepared for the sustained release of pioglitazone. Moreover, mouse bone marrow-derived macrophages (BMDM) were cultured with the pio-MS to evaluate the anti-inflammatory polarization effect on the BMDM.
2. Materials
2.1. Materials
Pioglitazone was purchased from LKT Laboratories, Inc., St. Paul, MN. Poly (L-lactic (LA) -co-glycolic acid (GA)) (PLGA) (weight averaged molecular weight 20,000, LA and GA component ratio 1 : 1) and NH4Cl were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan. Dichloromethane, sodium lauryl sulfate, NaHCO3, EDTA and penicillin-streptomycin were purchased from Nacalai Tesque, Inc., Kyoto, Japan. Poly (vinyl alcohol) (PVA) (polymerization degree = 1,000, saponification degree = 86–90%) was kindly supplied from Japan Vam & Poval Co. Ltd., Osaka, Japan. Phosphate buffered-saline solution (PBS) was purchased from Nissui Pharmaceutical Co. Ltd., Tokyo, Japan.
2.2. Preparation of PLGA microspheres incorporating pioglitazone (pio-MS)
Pioglitazone (5 mg) was dissolved in 3 ml of dichloromethane by stirring 1 hr at 50 °C. PLGA (100 mg) was dissolved in the pioglitazone solution. The resulting solution (RS) was poured into PVA aqueous solution (pio-MS0.5, pio-MS21 : 1.5 ml of RS in 20 ml of 2 wt% PVA aqueous solution, pio-MS40, pio-MS190, MS40 : 3 ml of RS in 300 ml of 0.3 wt% PVA aqueous solution). For the preparation of microspheres with a diameter less than 1 μm, the solution mixture (21.5 ml) was sonicated for 60 sec using a UD-21P ultrasonic generator (Tomy Seiko Co. Ltd., Tokyo, Japan). The emulsion of pioglitazone/PLGA solution in PVA solution was stirred (pio-MS0.5, pio-MS21 : 1000 rpm, pio-MS40, MS40 : 370 rpm, pio-MS190 : 180 rpm) at 25 °C overnight until dichloromethane was completely evaporated. By changing the stirring rate, four types of microspheres (pio-MS) with different sizes were prepared. The pio-MS prepared were washed several times with double-distilled water (DDW) by centrifugation (5000 rpm, 10 min, 4 °C) and freeze-dried to obtain pio-MS. The pio-MS were resolved in dichloromethane, and mixed with PBS followed by a vigorous shaking to allow pioglitazone to extract into PBS phase. The drug concentration of PBS was determined by high performance liquid chromatography (HPLC; Prominence, Shimazu Corp., Kyoto, Japan) equipped with one pump, an auto sampler, and an ultra violet (UV) detector. A calibration curve was prepared for determined amount of pioglitazone based on the UV absorbance peak area at 266 nm. The calibration curve was used to estimate the amount of pioglitazone incorporated.
The size of pio-MS was evaluated by observing with optical microscopy (BZ-X710, KEYENCE, Osaka, Japan) or scanning electron microscopy (SEM) (SU-3500, Hitachi Ltd., Tokyo, Japan). For the SEM observation, microspheres were fixed on an aluminum support with carbon-adhesive glue and coated with a thick coating of gold palladium (JSM 6701F; JEOL, Tokyo, Japan).
2.3. In vitro release test of pioglitazone from pio-MS incorporating pioglitazone
The pioglitazone release profiles from pio-MS were evaluated in vitro. Pio-MS were incubated and gently shaken in a tube containing 1.0 ml of PBS containing 1 wt% of sodium lauryl sulfate at 37 °C. PBS was collected at different time intervals, and the concentration of pioglitazone in the PBS was determined by HPLC equipped with one pump, an auto sampler, and an UV detector. A calibration curve was prepared for determined amount of pioglitazone based on the UV absorbance peak area at 266 nm. The calibration curve was used to estimate the amount of pioglitazone released. The pioglitazone release profile was calculated as follows: (cumulative amount of pioglitazone released)/(total released pioglitazone) × 100.
2.4. Preparation of mouse bone marrow-derived macrophages
All animal experiments were carried out in accordance with procedures approved by the Animal Experimentation Committee of Institute for Frontier Life and Medical Sciences, Kyoto University. Macrophages were harvested from the bone marrows of 7-week-old female C57BL/6n mice according to the protocol previously reported with slight modifications [26]. In brief, bone marrow cells were collected from the femurs and tibias of mice by flushing with PBS using a 10 ml syringe with a 27-gauge needle. After removal of red blood cells with red blood cell lysis buffer (4.15 g of NH4Cl, 0.5 g of NaHCO3 and 0.0186 g of EDTA were dissolved), the bone marrow cell suspension was cultured in Iscove's modified Dulbecco's medium (IMDM) (Thermo Fisher Scientific, Rockford, IL) supplemented with 20 vol % fetal bovine serum (FBS) (HyClone Laboratories, South Logan, UT), 50 ng/ml recombinant mouse macrophage colony stimulating factor (M-CSF) (BioLegend, San Diego, CA), and 1 wt% penicillin-streptomycin. After 3 days culture, penicillin-streptomycin was removed from the medium, and after further 6 days culture, the cells were used as bone marrow-derived macrophages (BMDM) in the subsequent experiments.
2.5. Evaluation of arginase activity and IL-10 secretion for BMDM cultured with free pioglitazone and microspheres
BMDM (3 × 105 cells/well) were seeded on conventional 12-well multi-dish culture plates (Corning Inc., Kennebunk, ME) and cultured for 24 hr with a culture medium composed of IMDM supplemented with 20% FBS and 50 ng/ml M-CSF followed by replacement of medium in which pio-MS, pioglitazone dissolved in dimethyl sulfoxide, PLGA microspheres (MS40) or the mixture of pioglitazone and MS40 were suspended and cultured for 48 hr. Then, the cells were used to evaluate the arginase activity by Arginase Assay Kit (BioAssay Systems, Hayward, CA) and the culture supernatants were collected to evaluate the level of secreted interleukin-10 (IL-10) by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc., Minneapolis, MN).
2.6. Real-time qPCR
For real-time qPCR, total RNA was extracted from BMDM cultured with pio-MS, pioglitazone, MS or mixture of pioglitazone and MS in the above-described method with RNeasy mini kit (Takara Bio Inc., Shiga, Japan). The reverse transcription (RT) reaction was carried out using SuperScript VILO (Thermo Fisher Scientific, Rockford, IL), and the following primers were used to amplify the target genes: PPARγ forward: 5′-ATCTACACGATGCTGGC-3′, reverse: 5′-GGATGTCCTCGATGGG-3’; STAT6 forward: 5′-TGA GGT GGG GAC CAG CCG G -3′,reverse: 5′-GTG ACC AGG ACA CAC AGC GG -3’; β-actin forward: 5′-TTTCCAGCCTTCCTTCTTGG-3′, reverse: 5′-TGGCATAGAGGTCTTTACGGATG-3’. Quantitative PCR was performed using an Applied Biosystems 7500 (Thermo Fisher Scientific, Rockford, IL). The expression levels of the target genes were standardized by β-actin.
2.7. Observation of macrophages by scanning electron microscopy
BMDM cultured with pio-MS, pioglitazone, MS or the mixture of pioglitazone and MS in the above-described method were washed with PBS and fixed with 2.5% glutaraldehyde in PBS at 4 °C for one night. SEM observation was performed on samples dried in a critical point with t-butyl alcohol and coated with a thin layer of gold-palladium.
2.8. Statistical analysis
All the data represented the results of three independent experiments. The data were expressed as the mean ± standard deviation (SD). The data were statistically analyzed by Tukey–Kramer paired comparison test. A p value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. Characteristics of PLGA microspheres incorporating pioglitazone
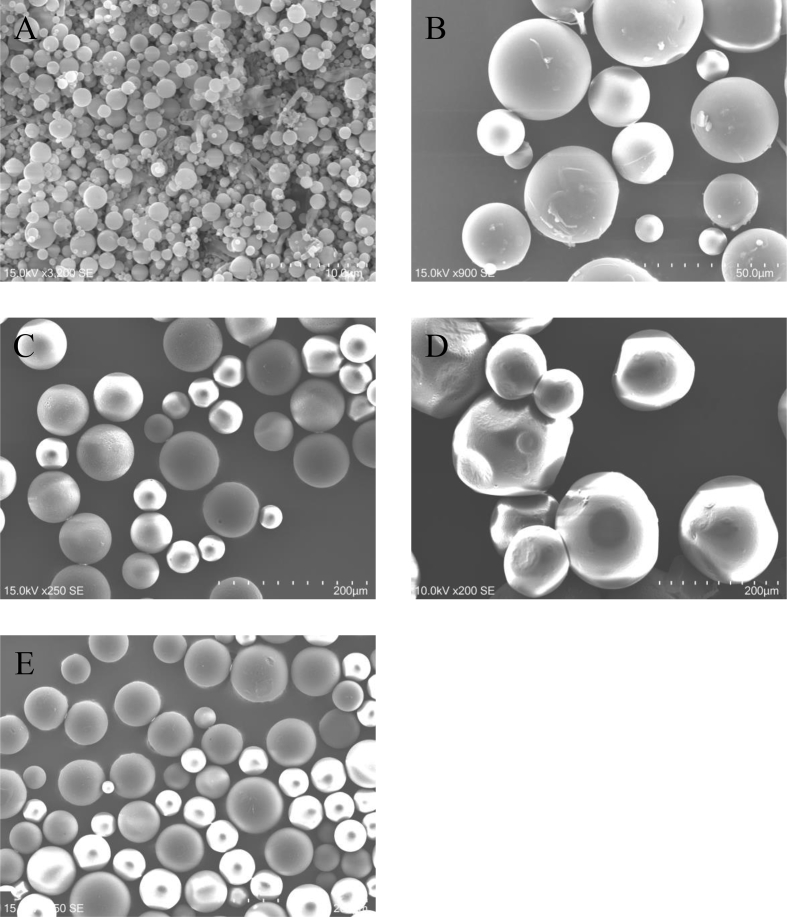
Table 1 summarizes the size of PLGA microspheres incorporating pioglitazone (pio-MS) and pioglitazone-free PLGA microspheres (MS). Fig. 1 shows the scanning electron micrographs of pio-MS0.5, pio-MS21, pio-MS40, pio-MS190, and MS40. The drug loading efficiency was 5.1 ± 0.2 μg/mg MS, irrespective of the MS type. The microspheres were all spherical with a smooth surface, irrespective of the size.
Table 1.
Characterization of PLGA microspheres incorporating pioglitazone.
| Code | Average diameters (μm) |
|---|---|
| pio-MS0.5 | 0.53 ± 0.26a) |
| pio-MS21 | 21.0 ± 10.3 |
| pio-MS40 | 40.5 ± 12.7 |
| pio-MS190 | 190 ± 27.4 |
| MS40b) | 42.3 ± 9.29 |
Average ± SD.
Pioglitazone-free empty MS.
Fig. 1.
Scanning electron micrographs of pio-MS0.5 (A), pio-MS21 (B), pio-MS40 (C), pio-MS190 (D), and MS40 (E).
3.2. Pioglitazone release profiles from pio-MS
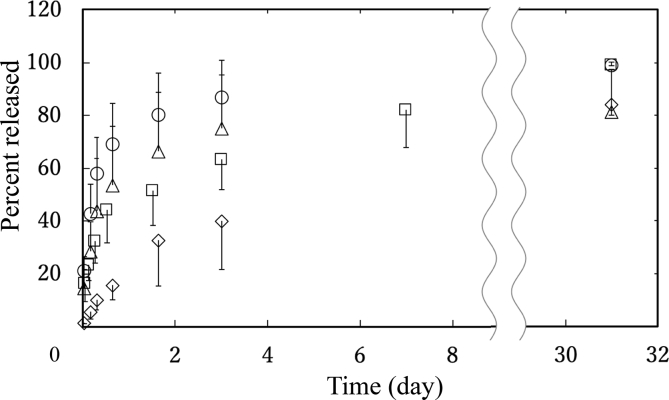
Fig. 2 shows the time profile of pioglitazone release from pio-MS. Pioglitazone was released from every pio-MS, irrespective of the size. Pioglitazone was released from pio-MS faster as the size of pio-MS decreased.
Fig. 2.
In vitro release profiles of pioglitazone from pio-MS0.5 (○), pio-MS21 (▵), pio-MS40 (□), and pio-MS190 (◊).
3.3. Arginase activity for BMDM cultured with free pioglitazone, pio-MS, MS40, the mixture of MS40 and free pioglitazone
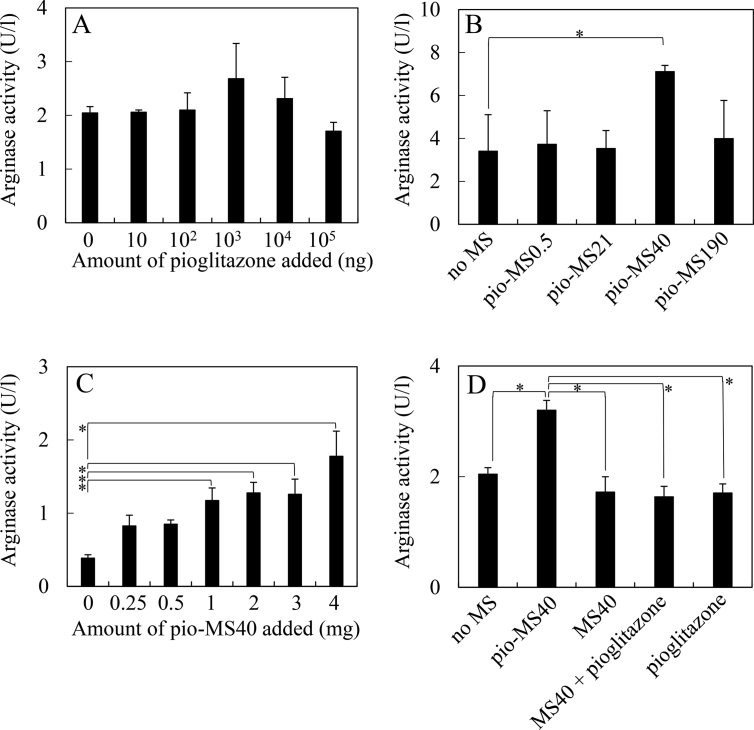
Fig. 3A shows the arginase activity for BMDM cultured with pioglitazone. The arginase was selected as one of M2 macrophage markers. The arginase activity did not change by increasing the amount of free pioglitazone added. Fig. 3B shows the arginase activity for BMDM cultured with pio-MS with different sizes. Pio-MS40 increased the arginase activity to a significant extent compared with free pioglitazone. Moreover, the arginase activity tended to increase by increasing the amount of pio-MS40 added (Fig. 3C). MS40 or the mixture of MS40 and pioglitazone did not increase the arginase activity (Fig. 3D).
Fig. 3.
Arginase activity of BMDM 48 h after cultured with pioglitazone, pio-MS, MS40, and the mixture of MS40 and pioglitazone. BMDM were cultured with different amounts of free pioglitazone (A), pio-MS (2 mg) with different sizes (B), different amounts of pio-MS40 (C), and pio-MS40 (2 mg), MS40 (2 mg), MS40 (2 mg) + free pioglitazone (100 mg), and free pioglitazone (100 mg) (D). *, p < 0.05; significant difference between the two groups.
3.4. IL-10 secretion for BMDMs cultured with free pioglitazone, pio-MS, MS40, the mixture of MS40 and free pioglitazone
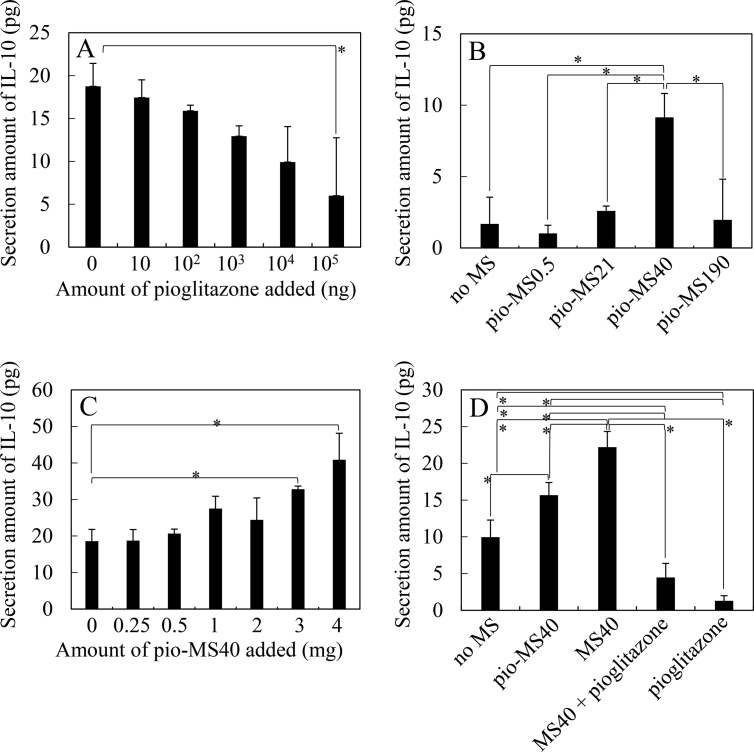
Fig. 4A shows the IL-10 secretion for BMDM cultured with pioglitazone. The IL-10 was used as another M2 macrophage marker. The IL-10 secretion decreased by increasing the amount of free pioglitazone added. Fig. 4B shows the secretion of IL-10 for BMDM cultured with pio-MS with different sizes. Pio-MS40 increased the secretion of IL-10 to a significant extent compared with other pio-MS. Moreover, the IL-10 secretion tended to increase by increasing the amount of pio-MS40 added (Fig. 4C). On the other hand, MS40 significantly increased the secretion of IL-10 although free pioglitazone and the mixture of MS40 and free pioglitazone did not increase the secretion of IL-10 (Fig. 4D).
Fig. 4.
IL-10 amount of secreted from BMDM 48 hr after cultured with pioglitazone, pio-MS, MS40, and the mixture of MS40 and pioglitazone. BMDM were cultured with different amounts of free pioglitazone (A), pio-MS (2 mg) with different sizes (B), different amounts of pio-MS40 (C), and pio-MS40 (2 mg), MS40 (2 mg), MS40 (2 mg) + free pioglitazone (100 mg), and free pioglitazone (100 mg) (D). *, p < 0.05; significant difference between the two groups.
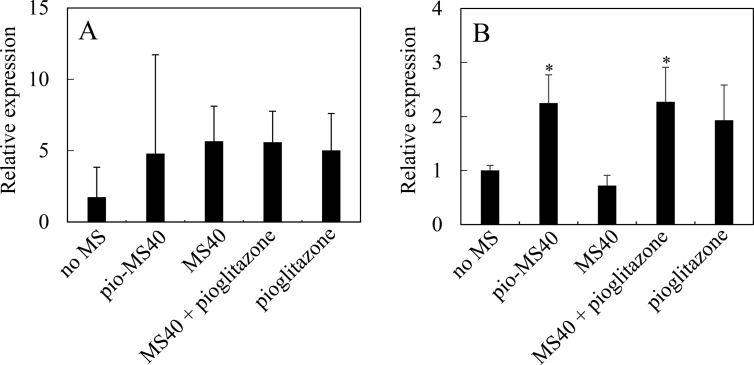
3.5. Effect of pio-MS on gene expression in BMDMs
When pioglitazone acts on the cell, PPARγ and STAT6 of intracellular signals are known to be up-regulated. In this study, their mRNA expression was examined to check whether or not pioglitazone is responded by the cell. Fig. 5 shows the mRNA level of PPARγ and STAT6 for BMDMs cultured with pio-MS40, MS40, the mixture of MS40 and free pioglitazone, and free pioglitazone. As shown in Fig. 5B, pio-MS40, and the mixture of MS40 and free pioglitazone significantly increased the STAT6 expression.
Fig. 5.
Gene expression of PPARγ (A) and STAT6 (B) for BMDM 48 hr after cultured with pio-MS40 (2 mg), MS40 (2 mg), MS40 (2 mg) + pioglitazone (100 mg) or pioglitazone (100 mg). *, p < 0.05; significant difference against the value of no MS.
3.6. Morphology of BMDMs after culture with pio-MS
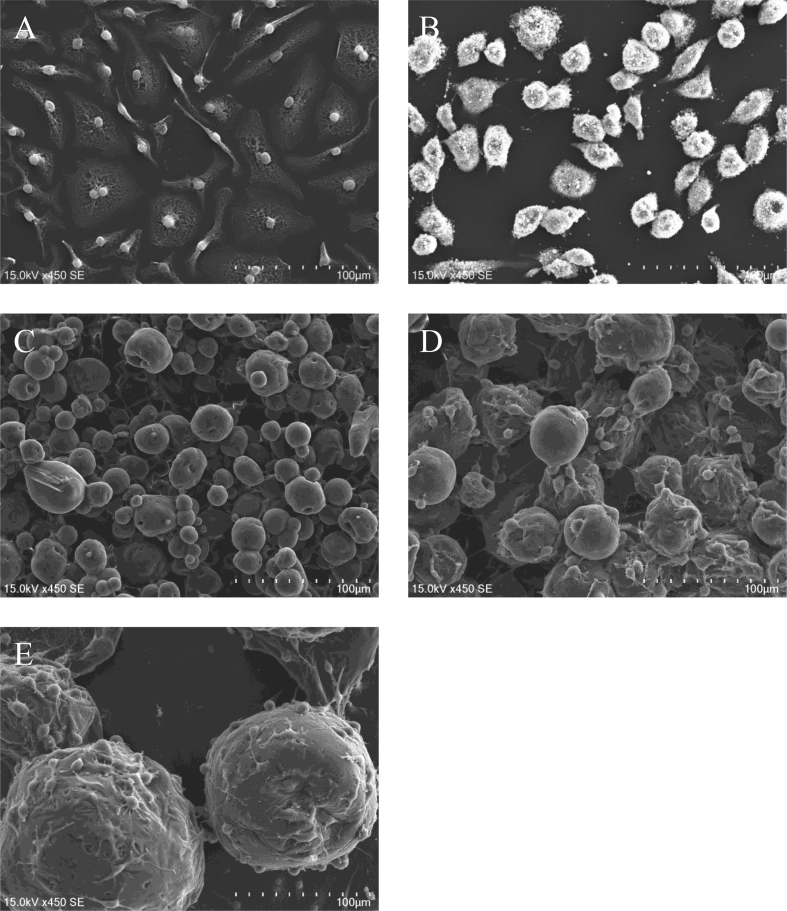
Fig. 6 shows the SEM photographs of BMDM 48 hr after cultured without MS, and with pio-MS0.5, pio-MS21, pio-MS40, and pio-MS190. For the pio-MS40 and pio-MS190, a lot of cells were adhered to the surface of microspheres. There was no significant difference in the number of cells adhered in unit surface between pio-MS40 and pio-MS190. On the other hand, the specific surface area of pio-MS40 was 5 times as large as that of pio-MS190 (Table 2).
Fig. 6.
Scanning electron micrographs of BMDM 48 hr after cultured without MS (A), and with pio-MS0.5 (2 mg) (B), pio-MS21 (2 mg) (C), pio-MS40 (2 mg) (D), and pio-MS190 (2 mg) (E).
Table 2.
Number of cells adhered on surface of BMDM 48 hr after culture with pio-MS40 and pio-MS190.
| Code | Number of adhered cells on the microspheres surface (cells/mm2) | Specific surface area |
|---|---|---|
| pio-MS40 | 115 ± 8.25a) | 1 |
| pio-MS190 | 84.2 ± 76.6 | 0.211 |
Average ± SD.
4. Discussion
The present study demonstrates that PLGA microspheres incorporating pioglitazone with the diameter of 40 μm modified an anti-inflammatory function of macrophages. Pioglitazone was released with time from every pio-MS with different sizes and the pioglitazone release became faster as the size of pio-MS decreased (Fig. 2). This can be explained by terms of MS specific surface area. It is conceivable that the specific surface area of MS increased with a decrease in the MS size, leading to the enhanced surface to release out pioglitazone. The enhanced surface area would fasten the pioglitazone release.
To induce the phenotype modification of macrophages, we used the pioglitazone of a PPARγ agonist which has an ability to change the macrophages function. It is demonstrated that the activation of PPARγ signaling plays a protective role in the tissue remodeling and wound repair by reducing oxidative stress and inflammation [27]. The local administration of rosiglitazone of another PPARγ agonist induced anti-inflammatory responses [28] and increased the expression of M2-specific marker [29]. Among the PPARγ agonists, pioglitazone is commercially available and one of the strong modulators for PPARγ. It has been clinically used as a diabetic's drug. Based on that, pioglitazone is a good candidate to experimentally confirm the function to modify the macrophages M1/M2 ratio aiming at the clinical application. The present study indicates that the PLGA microspheres incorporating pioglitazone enhanced an anti-inflammatory modification of macrophages phenotype.
To evaluate the effect of pioglitazone on the macrophage phenotype, BMDM were cultured with free pioglitazone to evaluate the arginase activity and the secretion of IL-10. Both the arginase activity and IL-10 are known as M2 macrophage markers. Free pioglitazone did not increase both the M2 macrophage markers. However, the IL-10 level decreased with an increase in pioglitazone concentration. The reason is not clear at present. On the other hand, the arginase activity and the secretion of IL-10 for BMDM were significantly increased by being cultured with pio-MS40. The mixture of free pioglitazone and MS40 did not affect the BMDM function, similarly to free pioglitazone and MS40. One point to be noted is that the arginase activity and IL-10 secretion for the control group varied from experiments to experiments. This may be due to the difference or variation in the nature of BMDM primarily isolated and prepared. The difference in the cell nature is often observed, especially for the cells isolated. However, in this study, the significant difference in the values between the experimental (pio-MS40) and control groups was reproducibly observed. This indicates that the pio-MS40 have an influence on the macrophages function. It is apparent from Fig. 5 that pioglitazone increased the STAT6 gene expression, irrespective of the addition way of pioglitazone in the free or released type. It is reported that the STAT6 transcription factor is a facilitator of PPARγ-regulated gene expression in macrophages [30]. This experimentally confirms that pioglitazone released functions PPARγ. Taken together, we can say with certainly that the sustained release of pioglitazone from PLGA microspheres was effective in promoting macrophage modification.
To consider the reason why the pio-MS40 had a positive influence on the M2 macrophages modification, SEM observation was carried out. As shown in Fig. 6B, the pio-MS0.5 were uptaken by macrophages. It is possible that an excessive uptake of microspheres physiologically weakens BMDM. On the other hand, a lot of macrophages adhered on the surface of pio-MS40 and pio-MS190 (Fig. 6D and E). No significant difference in the number of adhesive cells was observed for the two pio-MS (Table 2). However, the specific surface area of pio-MS40 adhering to cells was 5 times as large as that of pio-MS190. It means that pio-MS40 interact with cells more than pio-MS190. In addition, the morphology of cells was different on macrophages among pio-MS with different sizes. The change in the cell morphology may affect the macrophages response and functions. Moreover, the amount of pioglitazone released from the pio-MS40 was larger than that from the pio-MS190. The more interaction with BMDM and the larger and appropriate amount of pioglitazone released would permit the pio-MS40 to induce an anti-inflammatory phenotype of macrophages more effectively.
5. Conclusions
Pioglitazone was released from pio-MS, although the release profile depended on the type of pio-MS. The pio-MS40 with 40 μm average diameters enhanced the arginase activity and IL-10 secretion for BMDM, which means the induction of an anti-inflammatory phenotype macrophages. The pio-MS40 were promising to induce an anti-inflammatory polarization in macrophages.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Gordon S., Taylor R.P. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A., Sozzani S., Locali M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 3.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–959. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 5.Brown L.F., Yeo K.T., Berse B., Yeo T.K., Senger D.R., Dvorak H.F. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;175:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh T.J., DiPietro L.A. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak M.L., Koh T.J. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Brown B.N., Ratner B.D., Goodman S.B., Amar S., Badylak S.F. Macrophage polarization: an opportunity for improve outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouhlel M.A., Derudas B., Rigarnonti E., Dievart R., Brozek J., Haulon S. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabol. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Chinetti G., Fruchart J.C., Staels B. Peroxisome proliferator-activated receptors: new targets for the pharmacological modulation of macrophage gene expression and function. Curr Opin Lipidol. 2003;14:459–468. doi: 10.1097/00041433-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Chinetti G., Lestavel S., Fruchart J.C., Clavey V., Steals B. Peroxisome proliferator activated receptor alpha reduces cholesterol esterification in macrophages. Circ Res. 2003;92:212–217. doi: 10.1161/01.res.0000053386.46813.e9. [DOI] [PubMed] [Google Scholar]
- 13.Welch J.S., Ricote M., Akiyama T.E., Gonzalez F.J., Glass C.K. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. PNAS USA. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehrke M., Lazar M.A. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y., Kim C.S., Saylor D.M., Koo D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: a review of experiments and theories. J Biomed Mater Res B Appl Biomater. 2016;105:1692–1716. doi: 10.1002/jbm.b.33648. [DOI] [PubMed] [Google Scholar]
- 16.Bose R.J., Lee S.H., Park H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. J Biomed Mater Res B Appl Biomater. 2016;20:34. doi: 10.1186/s40824-016-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredenberg S., Wahlgen M., Reslow M., Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 19.Allison S.D. Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic)acid microparticle drug delivery systems. J Pharm Sci. 2007;97:2022–2035. doi: 10.1002/jps.21124. [DOI] [PubMed] [Google Scholar]
- 20.Hines D.J. Poly(lactic-co-glycolic) acid−controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. 2013;30:257–276. doi: 10.1615/critrevtherdrugcarriersyst.2013006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 22.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Gentile P., Chiono V., Crmagnola I., Hatton P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-Based biomaterials for bone tissue engineering. Mol Sci. 2014;15:3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mir M., Ahmed B., Rehan A. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf, B. 2017;159:217–231. doi: 10.1016/j.colsurfb.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S., Parmar A., Kori S., Sandhir R. PLGA-based nanoparticles: a new paradigm in biomedical applications. Trac Trends Anal Chem. 2016;80:30–40. [Google Scholar]
- 26.Kim Y.H., Tabata Y. Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell-derived factor-1 and a macrophage recruitment agent enhances wound closure. J Biomed Mater Res A. 2015;104:942–956. doi: 10.1002/jbm.a.35635. [DOI] [PubMed] [Google Scholar]
- 27.Wada K., Kamisaki Y. Anti-inflammatory effect of PPARgamma agonists: basics and clinical applications. Nihon Rinsho. 2010;68:278–283. [PubMed] [Google Scholar]
- 28.Takahashi Y., Hasegawa-Moriyama M., Sakurai T., Inada E. The macrophage mediated effects of the peroxisome proliferator -activated receptor gamma agonist rosiglitazone attenuate tactile allodynia in the early phase of neuropathic pain development. Anesth Analg. 2011;113:398–404. doi: 10.1213/ANE.0b013e31821b220c. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa-Moriyama M., Ohnou T., Godai K., Kurimoto T., Nakama M., Kanmura Y. Peroxisome proliferator-activated receptor gamma agonist rosiglitazone attenuates postincisional pain by regulating macrophage polarization. Biochem Biophys Res Commun. 2012;426:76–82. doi: 10.1016/j.bbrc.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Szanto A., Balint B.L., Nagy Z.S., Barta E., Dezso B., Pap A. STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]