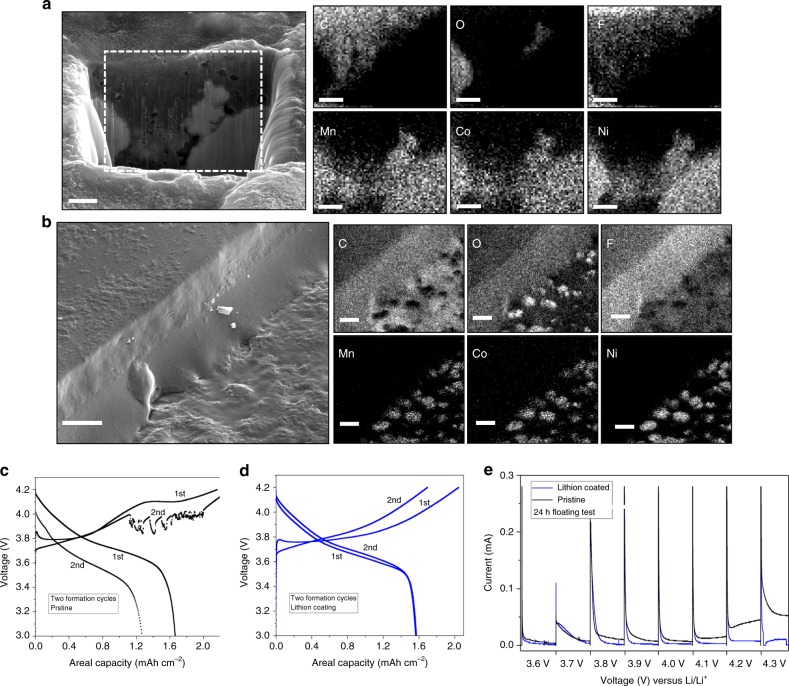
Fig. 3.
Immobilized anions at the cathode prevent glyme oxidation. a Cryo-SEM image of a cross-section of a Lithion-coated nickel manganese cobalt oxide (NCM) electrode obtained by focused ion beam milling. EDX mapping of different elements present in the cross-section is shown on the right. b Cryo-FIB/SEM images of the Lithion coated NCM electrode surface. A Lithion layer present on the NCM cathode is deliberately cracked during preparation to reveal its thickness. c Voltage profile of a lithium||NCM cell using the base electrolyte of diglyme–LiNO3–HFiP at C/10 rate; scale bars are 2 μm. d Voltage profile of Li||NCM cell using the same base electrolyte, however the cathode is coated with a layer of Lithion, operated at C/10; scale bars are 10 μm. e Electrochemical floating experiment in a Li||NCM cell. In these experiments, the voltage is fixed at different values ranging from 3.6 to 4.3 V for a period of 24 h and the current response measured to quantify the electrochemical stability of the electrolyte/electrode interphases over a range of potentials. The black curves represent results for uncoated NCM and blue are for Lithion-coated NCM electrodes