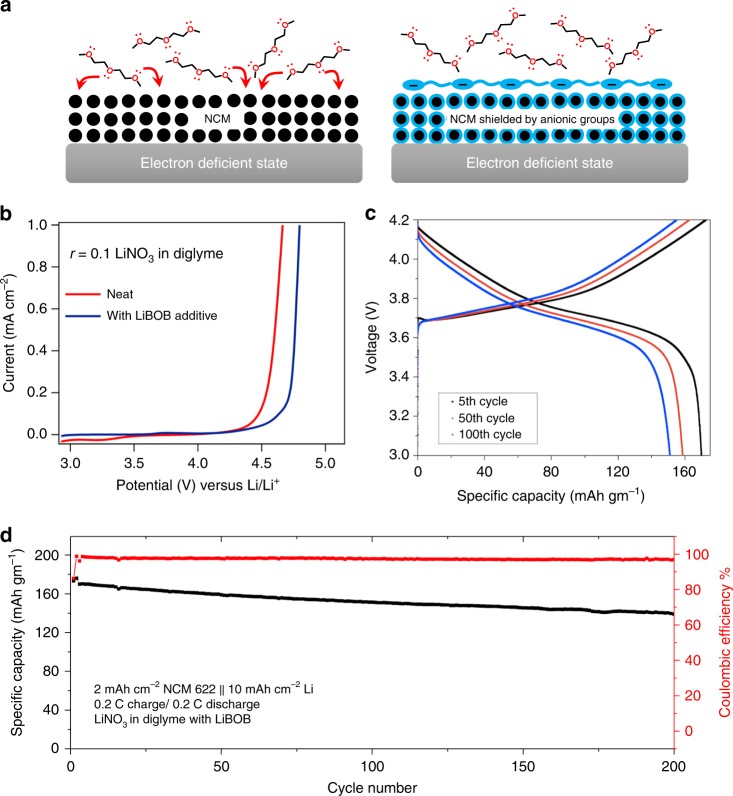
Fig. 5.
Enabling stable cycling of high voltage lithium battery with ether electrolytes. a Schematic showing the proposed mechanism by which oxidation of ethers is inhibited at a high-voltage CEI containing a layer of immobilized anions. b Potential-current diagram obtained from linear scan voltammetry in a 3-electrode cell in which Ag/AgCl is the reference electrode and stainless steel is used as both the working and counter-electrodes. The scan rate was 10 mV/s and diglyme–LiNO3–HFiP, with (blue) and without (red) 0.4 M LiBOB salt additive was used as the electrolyte. c Voltage profile for the 5th, 50th, and 100th charge and discharge cycles of a Li||NCM cell containing diglyme–LiNO3–HFiP electrolyte with 0.4 M LiBOB as salt additive. d Discharge capacity retention and coulombic efficiency over 200 cycles for a Li||NCM cell with diglyme–LiNO3–HFiP electrolyte with 0.4 M LiBOB as salt additive. Here, a 50 μm thick Li foil is used, and the anode to cathode (N:P) capacity ratio is 5