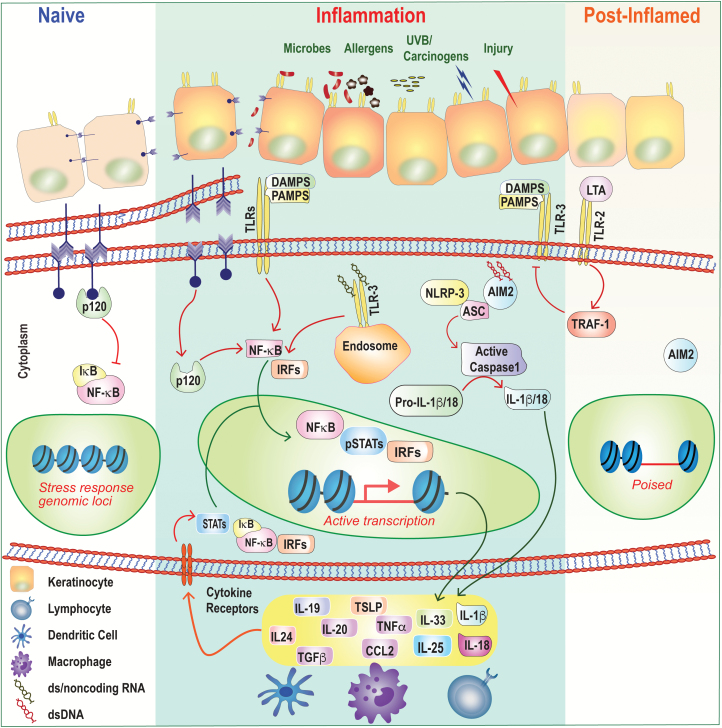
Fig. 2.
Epithelial inflammation and memory. Under homeostatic or ‘naive’ conditions, epithelial cells tether to each other by forming adherens junctions via E-cadherin and p120, which keeps inflammatory NF-κB activation at bay. These adherens junctions in the plasma membranes of the cells at the top-left of the figure (purple arrow heads) are shown in more detail below them. Barrier disruption and loss of cell-to-cell contact result in p120-mediated NF-κB nuclear translocation and in expression of inflammatory cytokines and chemokines. Epithelial cells express a number of PRRs that sense PAMPs and DAMPs including TLRs, NLRP3 and AIM2. Ligation of these receptors also induces downstream inflammatory transcriptional programs and/or activation of the inflammasome and processing of cytokines from the ‘pro’ to active forms. In some instances, ligation of TLRs can also be anti-inflammatory. Following skin injury, LTA from the commensal bacterium S. epidermidis dampens TLR3-mediated inflammation in a TRAF1-dependent manner. Inflammatory cytokines secreted by epithelial cells modulate immune cell function and can also signal autonomously into the epithelium to activate inflammatory transcription factors such as STATs, NF-κB and IRFs. Following resolution, epithelial progenitors retain a memory of inflammation by maintaining chromatin accessibility at key stress-response genes. These ‘poised’ loci enable a more rapid transcriptional response to secondary stimuli.