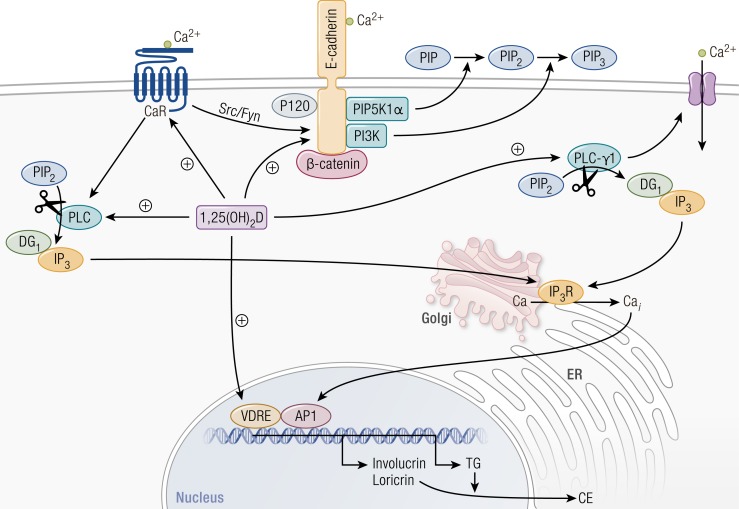
Figure 3.
Regulation of keratinocyte differentiation by calcium and 1,25(OH)2D. Calcium and 1,25(OH)2D interact to regulate keratinocyte differentiation at multiple steps. 1,25(OH)2D acts via its nuclear hormone receptor, VDR, to directly regulate gene transcription. Among the genes that it regulates are involucrin and loricrin, which encode major constituents of the cornified envelope (CE) as well as transglutaminase (TG) that crosslinks these proteins and others to form the CE. Although the effects of calcium on gene transcription do not appear to be direct, calcium is likely to act at least in part through protein kinase C, which phosphorylates and so activates transcription factors of the AP-1 family critical for the induction of these genes. Not shown is that 1,25(OH)2D also induces genes that encode enzymes that produce the long-chain lipids required for “waterproofing” the CE. 1,25(OH)2D also induces the calcium sensing receptor (CaR) that responds to extracellular calcium by activating phospholipase C (PLC). PLC, by cleaving phosphatidylinositol bisphosphate (PIP2), releases two important signaling molecules: diacylglycerol (DG) and inositol trisphosphate (IP3). The latter releases calcium from intracellular stores such as the endoplasmic reticulum (ER) and Golgi through the IP3 receptor (IP3R). DG works in conjunction with calcium to activate protein kinase C. 1,25(OH)2D induces both the β and γ forms of PLC, but calcium is required for their activation. The CaR also activates Src/Fyn, which phosphorylate the catenins, including β-catenin, to enable their binding to and formation of the E-cadherin complex in the membrane. Both calcium and 1,25(OH)2D are essential for the formation of this complex: 1,25(OH)2D induces E-cadherin, whereas calcium promotes its translocation to the membrane. Not shown is that α-catenin binds to β-catenin, linking the E-cadherin/catenin complex to the cytoskeleton, critical for cell migration. The E-cadherin/catenin complex also contains two enzymes, phosphatidylinositol phosphate 5 kinase 1α (PIP5K1α) and phosphatidylinositol 3 kinase (PI3K), that sequentially phosphorylate PIP to PIP2 to PIP3. PIP3 is the major activator of PLC-γ1 during keratinocyte differentiation, which in addition to promoting the cleavage of PIP2 to IP3 and DG also activates at least one of the calcium channels, TRP3. [Reproduced with permission from Bikle DD. Vitamin D, Calcium. and the Epidermis. In: Feldman D, Wesley Pike J, Bouillon R, et al., eds. Vitamin D. 4th ed. London: Academic Press; 2018:527-544.]