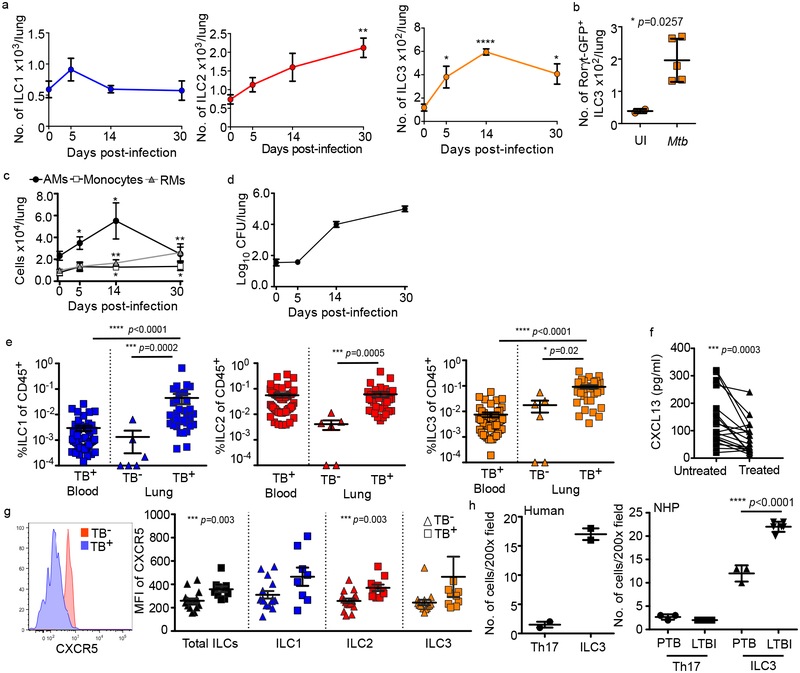
Figure 2. ILCs rapidly accumulate within lung tissues and are associated with lymphoid follicle containing granulomas.
(a-c) C57BL/6 (B6, n=5) mice or Rorγt-GFP (n=3–5) mice were aerosol infected with ~100 CFU Mtb. (a) Numbers of ILC1s, ILC2s, ILC3s in B6 mice and (b) number of ILC3s in Rorγt-GFP mice were quantified by flow cytometric analyses. (c) Numbers of AMs, monocytes, and recruited macrophages (RMs) were measured and quantified by flow cytometric analyses in B6 mice (n=5). (d) Bacterial burden was measured in the lungs of B6 mice by plating (n=5). Data shown as mean ± SEM (a) or mean ± SD (b-d). Where p-value not shown, *p<0.05, **p<0.01, ****p<0.0001. Significance by Student’s t-test (a-c). (e) Human ILC subsets were measured in TB infected lung tissue (TB+) compared to TB− control lung tissue, and in the circulation using flow cytometry. Significance by Kruskal-Wallis test with adjustments for multiple comparisons was carried out. (f) CXCL13 protein levels were measured in plasma from drug-susceptible TB subjects, and after 6 months of standard TB treatment (two-tailed Wilcoxon matched-pairs test). (g) CXCR5 expression was measured on circulating ILC subsets using flow cytometry. Significance by Mann-Whitney U test with correction for multiple comparisons; only significant p-values after correction shown. (h) ILC3 quantification in the FFPE lung sections from human, non-human primates (LTBI and PTB) was carried out by staining with CD3, B220 and RORγt and the number of RORγt+CD3− (ILC3) and RORγt+CD3+ (Th17) cells were counted and shown. Data shown as mean ± SD. Significance by Student’s t-test (h).