Figure 1. Pathophysiology of acute brain injury and therapeutic interventions.
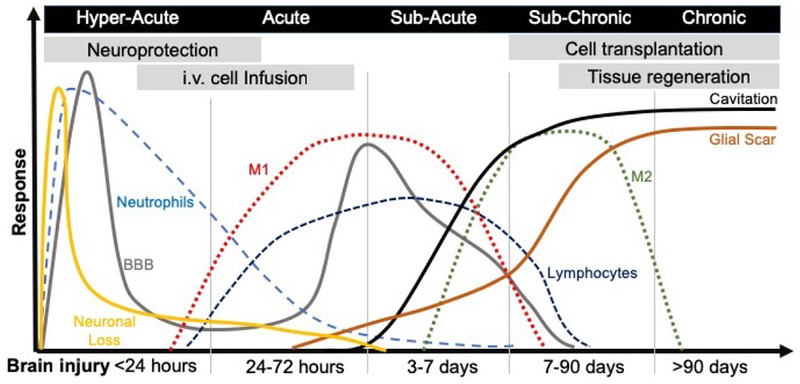
An acute insult to the brain through brute force or ischemia will lead to the rapid loss of neurons at the core of the infarct, this area will eventually cavitate and be sealed off by a glial scar. Apoptosis will further lead to neuronal death, as well as other cells, such as astrocytes, oligodendrocytes and endothelial cells (not illustrated here). Cell stress and cell death will provoke microglia to invoke an immune response that recruits neutrophils into brain tissue and produce a disruption in the blood brain barrier (BBB). The BBB will close again, but a second disruption will occur transitioning between the acute and subacute phase, again characterized by a major influx of peripheral immune cells, such as macrophages and lymphocytes. Most infiltrating macrophages are of a M1-type (i.e. pro-inflammatory) during the cell death and ECM clearance phase. However, as tissue is cleared these macrophages tend to polarize towards an M2-like (i.e. pro-repair) phenotype. It currently remains unclear if these are newly infiltrating cells or if ECM clearance in the brain promotes a shift towards a pro-repair phenotype. The implantation of inductive bioscaffolds needs to be considered against the backdrop of these pathophysiological events, but also in the context of other therapeutics and their time window. Endogenous in situ tissue engineering at present is best considered for treatment after a cavity formed in the sub-chronic phase. However, the use of bioscaffolds to change inflammatory events, promote neuronal survival, or neovascularization might be suitable for earlier interventions.
