Figure 4. ECM hydrogel implantation for endogenous in situ brain tissue engineering.
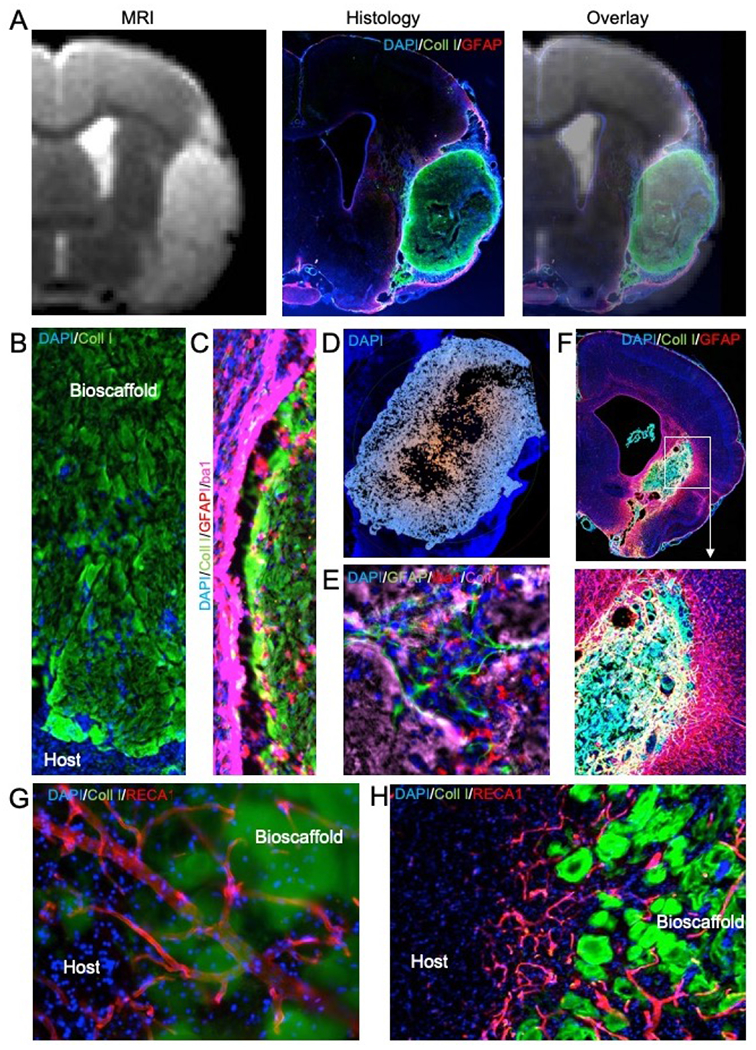
A. Magnetic resonance imaging (MRI)-based image guidance afforded an injection-drainage delivery of ECM hydrogel into a tissue cavity caused by a stroke. This afforded a complete coverage of the cavity with ECM bioscaffold, as evidence by the immunohistochemistry. An overlay of the pre-implantation MR image with 1-day post-mortem histology verifies the accuracy and efficiency of ECM bioscaffold delivery. B. Implantation of an ECM bioscaffold produced host cell invasion following the pattern of chain cell migration. C. The interface between the biomaterial and host tissue is essential to ensure an efficient invasion. D. A poor tissue-biomaterial interface or limited permeation of ECM derived signals into host tissue create invasion blind spots where no cell invasion occurs. Typically, invasion follows an outside-in migration pattern from all sides of the scaffold. E. Invading cells participate in constructive remodeling of the ECM hydrogel. In many cases, small pockets of tissue are forming inside the hydrogel, which gradually is being degraded by phagocytes. F. De novo tissue formed inside the cavity caused by a stroke after implantation of an ECM hydrogel (4 mg/mL). G. Blood vessels are being formed in the de novo tissue, with some vessels presented in the newly formed tissue and penetrating/passing through remnants of the ECM bioscaffold. H. In some cases, neovascularization passed in between ECM patches. The patches reflect a common pattern observed in degrading ECM bioscaffolds. Blood vessels supported newly forming tissue in between these patches that were remodeling the cavity and creating novel tissue.
