Figure 4.
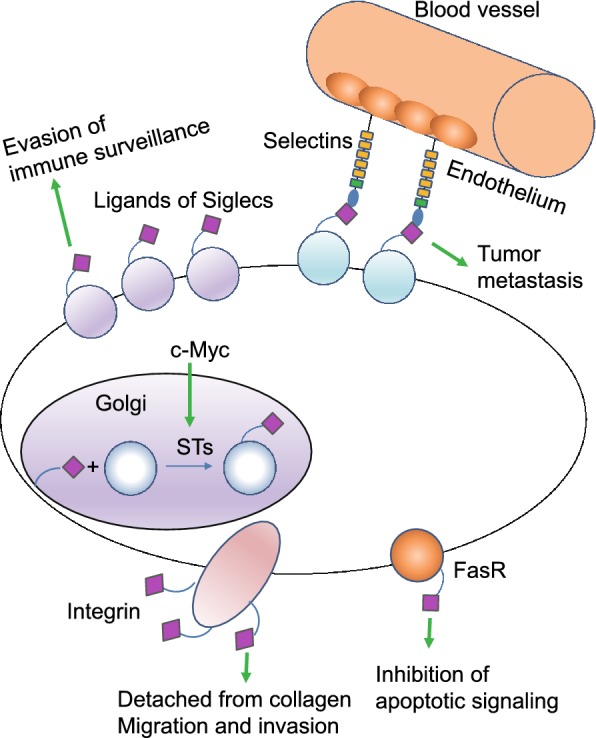
Sialylation is found to be aberrant in cancers compared to healthy controls, facilitating tumor growth and progression. In cancer cells, the proto-oncogene c-Myc, increase the expression of sialyltransferases (STs) in cancer cells. Therefore, the synthesis of sialylated glycans in the Golgi system by STs is enhanced. The aberrant high expression of sialylated glycans on Fas receptor (FasR) impairs the interaction between FasR and Fas, inhibiting apoptotic signaling transduction and preventing cancer cells from death. Moreover, increased sialylation on integrins can induce detachment from collagen, promoting cancer cell migration and tissue invasion. Cancer cell surfaces are enriched with glycans capped with SLX oligosaccharides which can interact with selectins, promoting cancer cells to adhere to and extravasate through the endothelium. Siglecs regulate immune surveillance of cancer and aberrant sialylation leads to Siglecs deficiency in cancer cells, preventing cancer cells from attack by immune system
