Table 2.
Structure-activity relationship of pyrrole and thiazole constituents of 1H-pyrrole-2-carboxamide compounds. IC50 values and max % inhibition were determined as in Table 1. DYN1 = dynamin-1.
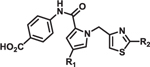 |
IC50 (μM) | Max % inhibition | ||||
|---|---|---|---|---|---|---|
| R1 | R2 | DRP1 | OPA1 | DYN1 | ||
| 8 | Cl | 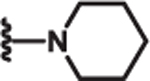 |
2.3 | >100 | >100 | 77 |
| 9 | Ph | 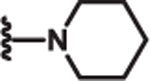 |
1.5 | >100 | >100 | l9 |
| 10 | Me | 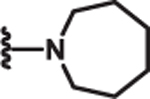 |
2.1 | >100 | >100 | 80 |
| 11 | Me | 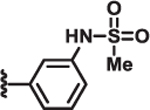 |
>100 | >100 | >100 | 6 |
