Fig. 7.
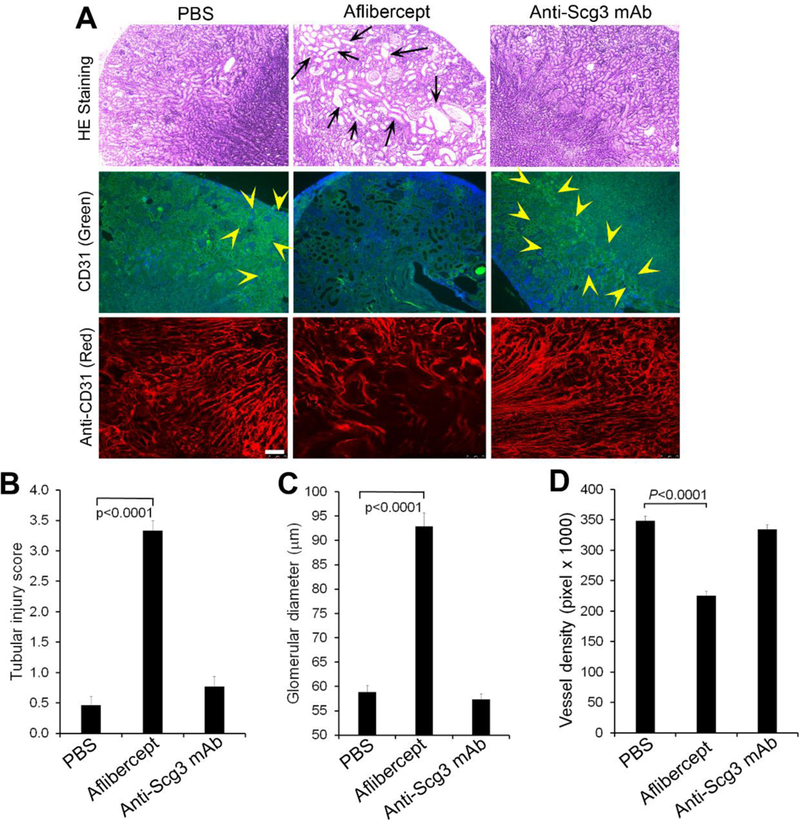
Anti-Scg3 mAb has no adverse on the developing kidney. Anti-Scg3 ML78.3 mAb or aflibercept (10 mg/Kg body weight) was injected i.p. into mice at P3, 5, 7, 9, 11 and 13. Kidneys were isolated at P15 and stained with H&E or anti-CD31 mAb. a Representative image of kidney H&E or CD31 Ab staining. Top row: H&E staining. Middle row: FITC-anti-CD31 mAb. Bottom row: Alexa Fluor 594-anti-CD31 mAb. Arrows indicate dilated tubules. Arrowheads indicate CD31-positive signals. b Quantification of tubular injury. c Quantification of glomerular dimeter. n=13 (PBS and anti-Scg3 mAb) and 16 (aflibercept). d Quantification of vessel density labeled with Alexa Fluor 594-anti-CD31 mAb. Bar=75 μm. n=8, ± SEM, one-way ANOVA test.
