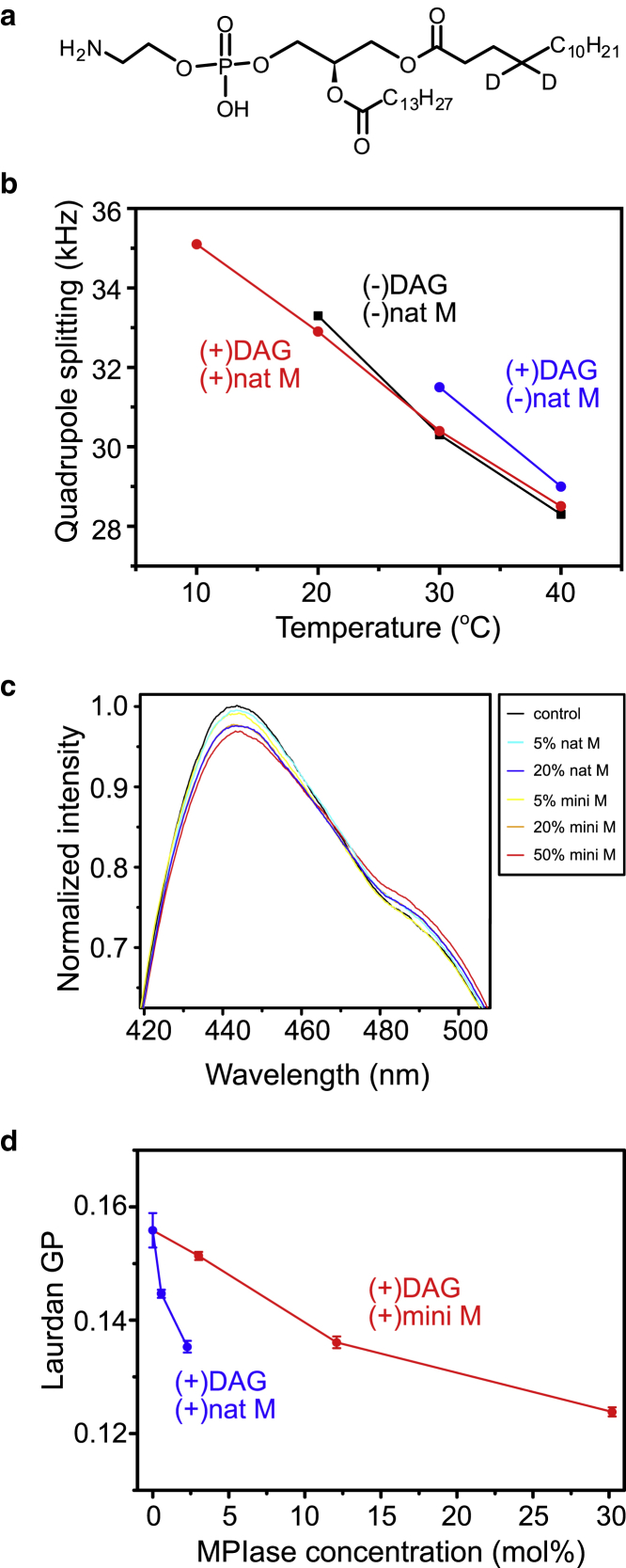
Figure 4.
(a) Molecular structure of selectively 2H-labeled DMPE (4-d2-DMPE) used in 2H NMR measurements. (b) Shown is the temperature dependence of the quadrupole splitting values in the 2H static spectra of EPL/2H-labeled DMPE/1,2-dimyristoyl-sn-glycero-3-phosphocholine (93.3:5:1.7 w/w/w) liposomes in the absence (black) and presence of DAG (5 wt%) (blue) and DAG (5 wt%)/natural MPIase (5 wt%) (red). As shown in Fig. S5, values for the control sample at 10°C and those for the DAG-containing sample at 10 and 20°C could not be measured by the broadening of peaks. (c) Shown are the emission spectra of laurdan in EPL/DAG (100:5 w/w), in the absence (black) and presence of 5 wt% (light blue), 20 wt% (blue) of natural MPIase, and 5 wt% (yellow), 20 wt% (orange), and 50 wt% (red) of mini-MPIase-3 at 30°C. Fluorescence intensity values were normalized by the peak area from 400 to 600 nm for each spectrum. Emission spectra were measured at a 360 nm excitation wavelength in all measurements. Data are presented as the means of three independent experiments. (d) Shown is the MPIase molar concentration dependence of laurdan GP values, GP = (I440 − I490)/(I440 + I490), for natural MPIase (blue) and mini-MPIase-3 (red) in the presence of 5 wt% DAG calculated from Fig. 4c. The error bars show the SD of three experiments.