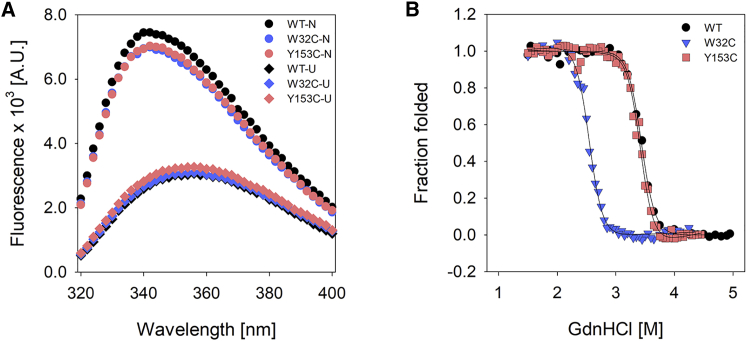
Figure 2.
Equilibrium folding profiles of PagP cysteine variants. (A) Shown are the fluorescence emission profiles of PagP wild-type (black) and representative mutants W32C (blue) and Y153C (red) derived from GdnHCl-induced chemical denaturation. Emission spectra are displayed for low (circles) and high (diamonds) denaturant concentrations, indicating the native (N) and unfolded (U) states of the protein. A.U., arbitrary units. (B) Shown are folding titrations displayed as folded fraction (fF) for representative mutants W32C and Y153C (see Fig. S5 for the complete data set). Folding profiles were acquired by monitoring the change in fluorescence emission intensity at a λem of 340 nm, corresponding to the λem-max of the folded protein. Data sets were fitted to the two-state equation (23) (fits are shown as solid lines). Data for wild-type PagP are shown in black. To see this figure in color, go online.