Abstract
Given the established roles of glucose‐dependent insulinotropic polypeptide (GIP) in promoting fat storage and bone formation, we assessed the contribution of GIP to obesity and osteopenia in ovariectomized mice with a gene encoding green fluorescent protein (GFP) inserted into the GIP locus, in which GIP was either reduced (GIPgfp/+) or absent (GIPgfp/gfp). In GIPgfp/gfp mice, weight gain, subcutaneous and visceral fat mass were reduced, and glucose intolerance was improved compared with wild‐type mice with the same magnitude of insulin responses. Cancellous bone mineral density and bone cortical thickness were reduced in GIPgfp/gfp mice compared with wild‐type mice. In GIPgfp/+ mice, weight gain, glucose intolerance and cancellous bone mineral density were not different from that of wild‐type mice. These results indicate that the total elimination of GIP ameliorates weight gain and adiposity in ovariectomized mice, but it enhances osteopenia, particularly in cancellous bone by partly suppressing bone formation.
Keywords: Glucose‐dependent insulinotropic polypeptide, Obesity, Ovariectomy
Introduction
Glucose‐dependent insulinotropic polypeptide (GIP) is a gut hormone released from enteroendocrine K cells that enhances insulin secretion after food intake1. The GIP receptor is expressed in pancreatic β‐cells, and other tissues including adipose tissue and bone2, 3, 4. We previously generated GIP‐deficient mice, and found that GIP deficiency protected the mice from high‐fat diet‐induced obesity and insulin resistance5, suggesting that blocking GIP signaling might be a strategy to treat obesity. However, mice lacking GIP showed signs of osteopenia, characterized by reduced bone volume, reduced number of trabeculae and increased osteoclast numbers5. Ovariectomy accelerates osteopenia and fat accumulation in the abdominal region6, 7, and leads to metabolic abnormalities, such as insulin resistance and dyslipidemia8, 9; however, the mechanisms remain unclear. To further investigate the role of GIP in fat, glucose and bone metabolism, we evaluated the effect of GIP deficiency on adipose tissue and bone metabolism in the setting of ovariectomy in mice.
Methods
Animal care and procedures were approved by Kyoto University Animal Care Committee (MedKyo16584).
GIP gene expression was reduced in C57BL/6J GIPgfp/+ mice or was entirely absent in GIPgfp/gfp mice compared with wild‐type (WT) mice, which were all housed as described previously5. Surgical ovariectomies (dorsal approach) were carried out on female WT, GIPgfp/+ and GIPgfp/gfp mice at the age of 8 weeks. Experiments were carried out on three separate cohorts of mice, each consisting of three groups of five to seven mice. Body fat mass, food intake along with energy expenditure and locomotor activity were measured as described previously10, 11. Oral glucose tolerance tests (OGTTs) were carried out at 17 and 37 weeks‐of‐age using 2 g/kg body weight glucose, and insulin tolerance tests were carried out at 24 and 40 weeks‐of‐age using 0.5 U/kg regular insulin as described previously10. Plasma insulin, total GIP and glucagon‐like polypeptide‐1 (GLP‐1) levels were measured using a mouse insulin enzyme‐linked immunosorbent assay kit (Shibayagi, Gunma, Japan), GIP enzyme‐linked immunosorbent assay kit (EMD Millipore Corporation, Billerica, MA, USA) and total GLP‐1 enzyme‐linked immunosorbent assay kit (Meso Scale Discovery, Rockville, MD, USA), respectively. Bone analysis by dual‐energy X‐ray absorptiometry and microcomputed tomography (μCT; LCT‐100M, Aloka, Tokyo, Japan), and the measurement of plasma osteocalcin and C‐terminal telopeptide of type I collagen using a mouse osteocalcin EIA kit (Biomedical Technologies Inc., Stoughton, MA, USA) and RatLaps™ EIA kit (Immunodiagnostic Systems Inc, Gaithersburg, MD, USA) were carried out at 16 weeks‐of‐age. The blood samples were collected from the tail vein without anesthesia.
All data are expressed as the mean ± standard error of the mean. Statistical analysis was carried out using one‐way anova with the Tukey–Kramer multiple comparison test. P‐values <0.05 were considered significant.
Results
Body weight gain after ovariectomies was tracked in cohort 2 (Figure 1a). The body weight of GIPgfp/gfp mice was significantly lower than WT mice from 25 weeks‐of‐age, but there was no difference between WT and GIPgfp/+ mice throughout the study. As expected, the uterus showed atrophy in all ovariectomized mice (data not shown). Both subcutaneous and visceral fat depots were reduced by ~40% in GIPgfp/gfp mice, but not significantly reduced in GIPgfp/+ mice compared with those in WT mice at 26 weeks‐of‐age in cohort 1 (Figure 1b). Lean body weight, food intake, locomotor activity and energy expenditure were not different among all three groups (Figure 1b‐e).
Figure 1.
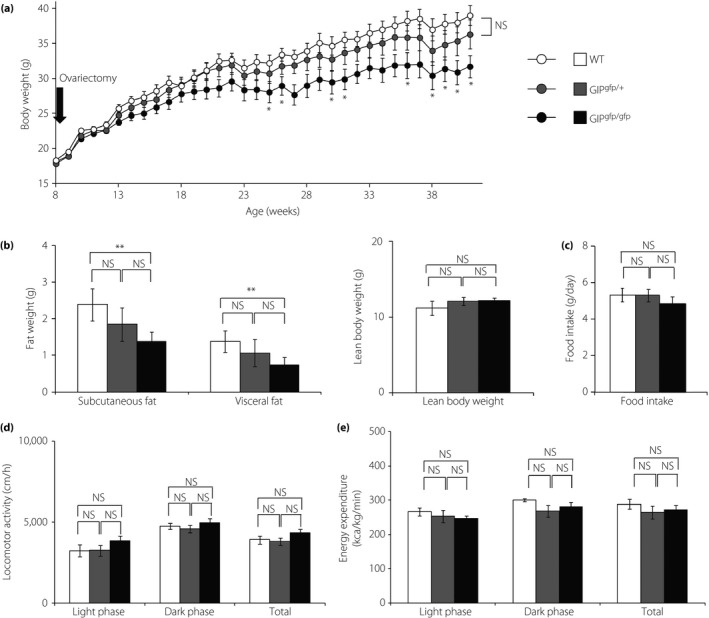
The phenotype of ovariectomized mice. (a) Body weight tracking after ovariectomies in cohort 2 (n = 7), (b) fat weight and lean body weight, (c) food intake, (d) locomotor activity, and (e) energy expenditure at 26 weeks‐of‐age for cohort 1 (n = 6). (a) *P < 0.05 compared with wild‐type mice (WT; white circles and bars). (b–e) *P < 0.05, **P < 0.01. GFP, green fluorescent protein; GIPgfp/+ green fluorescent protein inserted into the glucose‐dependent insulinotropic polypeptide locus, in which glucose‐dependent insulinotropic polypeptide locus was reduced (gray circles and bars); GIPgfp/gfp glucose‐dependent insulinotropic polypeptide locus was absent (black circles and bars); NS, not significantly different.
Blood glucose levels during OGTTs at 17 weeks‐of‐age in cohort 1 were not different (Figure 2a). Insulin levels were decreased at 30 min after glucose administration in GIPgfp/gfp mice compared with WT mice, but the area under the curves (AUC) of plasma insulin responses were not different (Figure 2b). The AUC of plasma GIP were under the detection level in GIPgfp/gfp mice, and the AUC of GIP responses were similar in WT and GIPgfp/+ (Figure 2c,g). By 37 weeks‐of‐age in cohort 2, blood glucose levels were significantly decreased in GIPgfp/gfp mice compared with WT, resulting in a lower AUC (Figure 2e). In contrast, insulin responses to oral glucose were not different among the three groups (Figure 2f). Plasma GLP‐1 levels during OGTT were not significantly different in WT and GIPgfp/gfp mice (15.81 ± 2.55 and 11.95 ± 6.26 pg/dL at 15 min after OGTT, respectively). There were no differences in glucose reduction in response to exogenous insulin administration among the three groups at either 24 or 40 weeks‐of‐age (Figures 2d,h). The ovariectomized WT mice showed GIP hypersecretion, obesity and insulin resistance compared with non‐ovariectomized WT mice (Figure S1).
Figure 2.
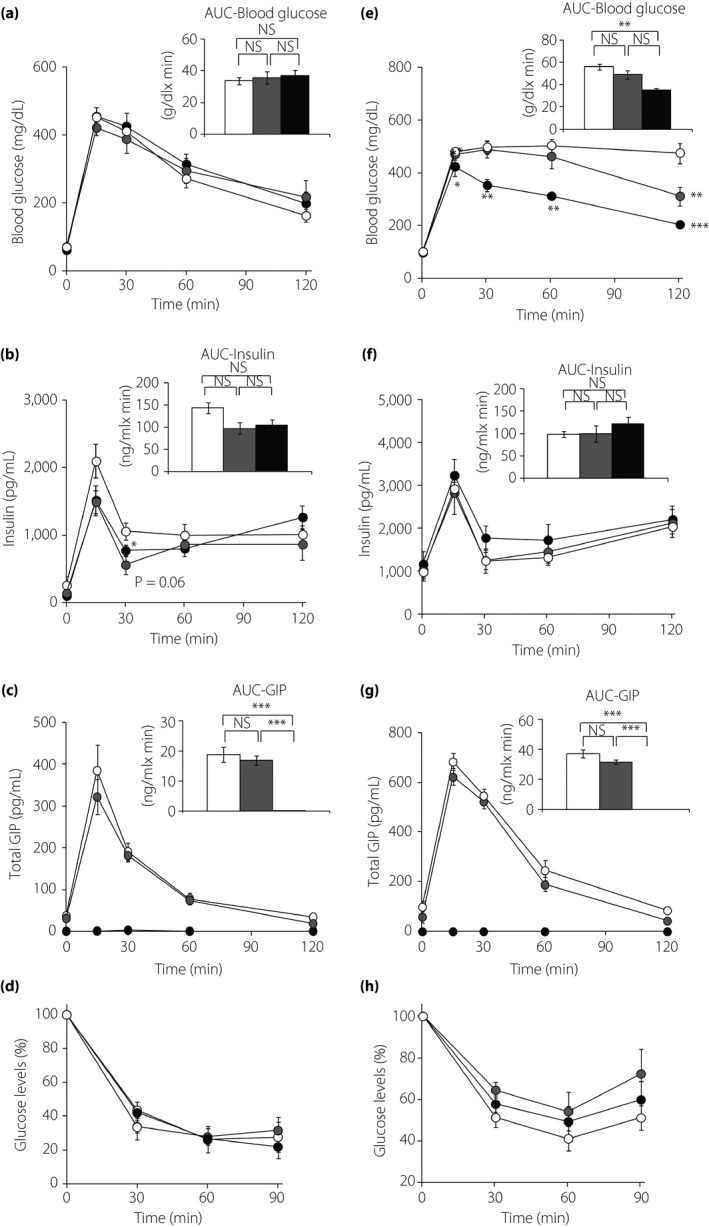
Oral glucose tolerance tests (OGTTs) and insulin tolerance tests (ITTs) in ovariectomized mice. OGTT and ITT in wild‐type (WT; white circles and bars), green fluorescent protein inserted into the glucose‐dependent insulinotropic polypeptide (GIP) locus, in which the GIP locus was reduced (GIPgfp/+; gray circles and bars) and absent (GIPgfp/gfp; black circles and bars) mice. OGTTs were carried out at (a–c) 17 weeks‐of‐age in cohort 1 (n = 6) and (e–g) 37 weeks‐of‐age in cohort 2 (n = 7). ITTs were carried out at (d) 24 weeks in cohort 1 (n = 4‐6) and (h) 40 weeks in cohort 2 (n = 7). (a,e) Blood glucose levels, (b,f) plasma insulin levels during OGTTs, (c,g) plasma total GIP levels during OGTTs and (d,h) blood glucose levels during ITTs as the percentage change from fasting glucose levels. The area under the curves (AUC) are shown in the upper right panel of each figure. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT. NS, not significantly different.
At 16 weeks‐of‐age in cohort 3, body length and bone mineral density measured by dual‐energy X‐ray absorptiometry, whole and cortical bone mineral density as determined by microcomputed tomography, and plasma C‐terminal telopeptide of type I collagen levels were not different between the three groups (Table 1). However, cancellous bone mineral density, cortical thickness and plasma osteocalcin levels were decreased by 64%, 50% and 38% in GIPgfp/gfp mice compared with WT mice, respectively. Cortical thickness and plasma osteocalcin levels were decreased by 43% and 27% in GIPgfp/gfp mice compared with GIPgfp/+ mice, respectively, whereas there was no difference in GIPgfp/+ mice compared with WT mice.
Table 1.
Bone analysis in ovariectomized mice
| WT | GIPgfp/+ | GIPgfp/gfp | |
|---|---|---|---|
| Body length (cm) | 9.4 ± 0.02 | 9.4 ± 0.06 | 9.4 ± 0.07 |
| Body weight (g) | 25.5 ± 0.82 | 25.8 ± 1.02 | 23.5 ± 0.72 |
| BMD (g/cm2) | 22.2 ± 1.1 | 23.6 ± 1.5 | 20.7 ± 1.3 |
| Whole BMD (mg/cm3) | 360.3 ± 28.2 | 325.5 ± 8.0 | 308.4 ± 19.6 |
| Cortical BMD (mg/cm3) | 358.9 ± 22.7 | 331.7 ± 9.3 | 332.8 ± 6.3 |
| Cancellous BMD (mg/cm3) | 287.8 ± 63.7 | 148.3 ± 14.3 | 104.4 ± 22.4* |
| Cortical thickness (cm) | 0.074 ± 0.008 | 0.066 ± 0.009 | 0.038 ± 0.001**, *** |
| Plasma osteocalcin (ng/mL) | 47.8 ± 3.1 | 40.1 ± 3.2 | 29.4 ± 2.1*, *** |
| Plasma CTx (ng/mL) | 16.9 ± 0.71 | 17.0 ± 0.53 | 17.9 ± 0.51 |
Data presented as the mean ± standard error of the mean. BMD, bone mineral density; CTx, C‐terminal telopeptide of type I collagen. *P < 0.05 versus wild‐type mice (WT). **P < 0.01 versus WT. ***P < 0.05 versus green fluorescent protein (GFP) inserted into the glucose‐dependent insulinotropic polypeptide (GIP) locus, in which the GIP locus was reduced (GIPgfp/+) and absent (GIPgfp/gfp) mice.
Discussion
Ovarian hormone deficiency increases abdominal fat, insulin resistance and osteopenia8, 9, 12. We investigated the role of GIP in a rodent ovariectomy model, and the combined effect of ovarian hormone deficiency and GIP deficiency. We found that weight gain, subcutaneous and visceral fat mass, cancellous bone mineral density, bone cortical thickness, and plasma osteocalcin levels were reduced in GIP knockout mice compared with WT mice. These results are consistent with previous findings in GIP receptor knockout mice, GIP receptor antagonists, chemical K‐cell ablation, and GIP antibody therapy showing the anabolic effect of GIP on adipose tissue5, 13, 14, 15 and bone16.
Although we did not detect significant changes in food intake or locomotor activity, we cannot exclude the possibility that small reductions in food intake or increase in locomotor activity contributed to reduced weight gain in GIPgfp/gfp mice. We also observed improved glucose intolerance in GIPgfp/gfp mice aged 37 weeks‐of‐age compared with WT mice with the same magnitude of insulin responses, whereas no difference was seen at 17 weeks‐of‐age. Perhaps the significantly lower body weight and lower visceral fat mass in GIPgfp/gfp mice compared with WT mice after 26 weeks‐of‐age might have contributed to these results. We have previously reported that GLP‐1 secretion remained unchanged in GIPgfp/gfp mice5, and the present study also showed no compensatory hypersecretion of GLP‐1 in the ovariectomized GIP‐deficient mice.
We did not detect any improvement in insulin sensitivity, which might have been expected with reduced fat accumulation, but we cannot exclude the possibility of subtle changes in insulin sensitivity that could not be detected by our whole‐body insulin tolerance tests. We speculate that glucose homeostasis is not dramatically impaired in the GIP‐deficient mice, because lower body weight and visceral fat mass improved insulin sensitivity.
The present study did not show any significant changes in glucose and bone metabolism in GIPgfp/+ mice compared with WT mice. These results were different from previous findings on partial reduction of GIP signaling5, 17. Although we used GIPgfp/+ mice in which GIP levels were reduced before ovariectomies (Figure S2), the levels of GIP at OGTT were similar to that of WT mice after ovariectomies. The mechanisms of how ovariectomy might influence GIP production in GIPgfp/+ mice are unknown, but potentially, changes in estrogen or gonadotropin hormones might alter GIP secretion.
Regarding the role of GIP on bone metabolism, reduced bone formation, decreased bone strength and bone quality have been reported in GIP receptor knockout mice18, 19, 20, 21, and conversely, GIP‐overexpressing transgenic mice showed increased bone mass22. Although there is a report of osteocalcin‐induced release of glucagon‐like peptide‐123, no report that GIP regulates osteocalcin directly exists as far as we know. Ovarian hormone deficiency induced osteopenia itself, but GIP deficiency enhanced osteopenia, particularly in cancellous bone by partly suppressing bone formation. We have to consider not only estrogen deficiency, but also elevated gonadotropins might contribute to bone metabolism. There are very few reports of the relationship between GIP and estrogen deficiency. In humans, plasma GIP levels were approximately twice as high in postmenopausal women as young premenopausal women24, and estrogen replacement therapy reduced plasma GIP levels in postmenopausal women25. We could investigate only a part of the relationship between GIP and estrogen in the present study, but the mechanism by which estrogen modulates GIP production requires further study.
In conclusion, the present study supports the concept that the total elimination of GIP might reduce weight gain and improve glucose metabolism, but could be associated with undesirable consequences on bone loss in the setting of ovariectomy in mice.
Disclosure
NI served as a medical advisor for Takeda, Taisho, GlaxoSmithKline and Mitsubishi Tanabe; lectured for MSD, Sanofi, Novartis, Dainippon Sumitomo, Kyowa Kirin and Mitsubishi Tanabe; and received payment for services, outside the submitted work. The other authors declare no conflict of interest.
Supporting information
Figure S1 | OGTT and ITT in ovariectomized WT (WT) mice and non‐ovariectomized WT (WT sham) mice (n = 4–6). Body weight of WT mice and WT sham mice were 44.2 ± 2.1 g and 28.2 ± 1.4 g, respectively (P < 0.01). *P < 0.05, **P < 0.01 compared to WT sham. GIP, glucose‐dependent insulinotropic polypeptide; HOMA‐IR, homeostasis model assessment of insulin resistance; WT, wild‐type. Data are expressed as means ± standard error of the mean.
Figure S2 | OGTT in female 9 weeks of age in WT, GIPgfp/+ and GIPgfp/gfp mice (n = 7). *P < 0.05, **P < 0.01 compared to WT. GIP, glucose‐dependent insulinotropic polypeptide; GFP, green fluorescent protein; WT, wild‐type. Data are expressed as means ± standard error of the mean.
Acknowledgments
The authors thank Mr Shoichi Asano for technical assistance. This work was supported by JSPS Core‐to‐Core Program, A. Advanced Research Networks; JSPS KAKENHI Grant Numbers JP18K16197; grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); Japan Society for the Promotion of Science; Ministry of Health, Labour, and Welfare; Ministry of Agriculture, Forestry and Fisheries; Japan Diabetes Foundation; Japan Association for Diabetes Education and Care; MSD life science foundation; and Japan Foundation for Applied Enzymology.
J Diabetes Investig 2019; 10: 909–914
References
- 1. Cho YM, Kieffer TJ. K‐cells and glucose‐dependent insulinotropic polypeptide in health and disease. Vitam Horm 2010; 84: 111–150. [DOI] [PubMed] [Google Scholar]
- 2. Usdin TB, Mezey E, Button DC, et al Gastric inhibitory polypeptide receptor, a member of the secretin‐vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993; 133: 2861–2870. [DOI] [PubMed] [Google Scholar]
- 3. Bollag RJ, Zhong Q, Phillips P, et al Osteoblast‐derived cells express functional glucose‐dependent insulinotropic peptide receptors. Endocrinology 2000; 141: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 4. Joo E, Harada N, Yamane S, et al Inhibition of Gastric inhibitory polypeptide receptor signaling in adipose tissue reduces insulin resistance and hepatic steatosis in high fat diet‐fed mice. Diabetes 2017; 66: 868–879. [DOI] [PubMed] [Google Scholar]
- 5. Nasteska D, Harada N, Suzuki K, et al Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high‐fat diet conditions. Diabetes 2014; 63: 2332–2343. [DOI] [PubMed] [Google Scholar]
- 6. Thurston RC, Sowers MR, Sternfeld B, et al Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women's health across the nation. Am J Epidemiol 2009; 170: 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Pelt RE, Gavin KM, Kohrt WM. Regulation of body composition and bioenergetics by estrogens. Endocrinol Metab Clin North Am 2015; 44: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003; 88: 2404–2411. [DOI] [PubMed] [Google Scholar]
- 9. Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 2004; 5: 197–216. [DOI] [PubMed] [Google Scholar]
- 10. Shimazu‐Kuwahara S, Harada N, Yamane S, et al Attenuated secretion of glucose‐dependent insulinotropic polypeptide (GIP) does not alleviate hyperphagic obesity and insulin resistance in ob/ob mice. Mol Metab 2017; 6: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park SY, Kim MJ, Kim YJ, et al Selective PCAF inhibitor ameliorates cognitive and behavioral deficits by suppressing NF‐κB‐mediated neuroinflammation induced by Aβ in a model of Alzheimer's disease. Int J Mol Med 2015; 35: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 12. Garnero P, Sornay‐Rendu E, Chapuy MC, et al Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 1996; 11: 337–349. [DOI] [PubMed] [Google Scholar]
- 13. Miyawaki K, Yamada Y, Ban N, et al Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002; 8: 738–742. [DOI] [PubMed] [Google Scholar]
- 14. McClean PL, Irwin N, Cassidy RS, et al GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high‐fat diet. Am J Physiol Endocrinol Metab 2007; 293: E1746–E1755. [DOI] [PubMed] [Google Scholar]
- 15. Boylan MO, Glazebrook PA, Tatalovic M, et al Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. Am J Physiol Endocrinol Metab 2015; 309: E1008–E1018. [DOI] [PubMed] [Google Scholar]
- 16. Bollag RJ, Zhong Q, Ding KH, et al Glucose‐dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol Cell Endocrinol 2001; 177: 35–41. [DOI] [PubMed] [Google Scholar]
- 17. McClean PL, Irwin N, Hunter K, et al (Pro(3))GIP[mPEG]: novel, long‐acting, PEGylated antagonist of gastric inhibitory polypeptide for obesity‐diabetes (diabesity) therapy. Br J Pharmacol 2008; 155: 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsukiyama K, Yamada Y, Yamada C, et al Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol 2006; 20: 1644–1651. [DOI] [PubMed] [Google Scholar]
- 19. Xie D, Cheng H, Hamrick M, et al Glucose‐dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover. Bone 2005; 37: 759–769. [DOI] [PubMed] [Google Scholar]
- 20. Mieczkowska A, Irwin N, Flatt PR, et al Glucose‐dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone 2013; 56: 337–342. [DOI] [PubMed] [Google Scholar]
- 21. Gaudin‐Audrain C, Irwin N, Mansur S, et al Glucose‐dependent insulinotropic polypeptide receptor deficiency leads to modifications of trabecular bone volume and quality in mice. Bone 2013; 53: 221–230. [DOI] [PubMed] [Google Scholar]
- 22. Xie D, Zhong Q, Ding KH, et al Glucose‐dependent insulinotropic peptide‐overexpressing transgenic mice have increased bone mass. Bone 2007; 40: 1352–1360. [DOI] [PubMed] [Google Scholar]
- 23. Mizokami A, Yasutake Y, Gao J, et al Osteocalcin induces release of glucagon‐like peptide‐1 and thereby stimulates insulin secretion in mice. PLoS ONE 2013; 8: e57375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ranganath L, Sedgwick I, Morgan L, et al The ageing entero‐insular axis. Diabetologia 1998; 41: 1309–1313. [DOI] [PubMed] [Google Scholar]
- 25. Sztefko K, Rogatko I, Milewicz T, et al Effect of hormone therapy on the enteroinsular axis. Menopause 2005; 12: 630–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | OGTT and ITT in ovariectomized WT (WT) mice and non‐ovariectomized WT (WT sham) mice (n = 4–6). Body weight of WT mice and WT sham mice were 44.2 ± 2.1 g and 28.2 ± 1.4 g, respectively (P < 0.01). *P < 0.05, **P < 0.01 compared to WT sham. GIP, glucose‐dependent insulinotropic polypeptide; HOMA‐IR, homeostasis model assessment of insulin resistance; WT, wild‐type. Data are expressed as means ± standard error of the mean.
Figure S2 | OGTT in female 9 weeks of age in WT, GIPgfp/+ and GIPgfp/gfp mice (n = 7). *P < 0.05, **P < 0.01 compared to WT. GIP, glucose‐dependent insulinotropic polypeptide; GFP, green fluorescent protein; WT, wild‐type. Data are expressed as means ± standard error of the mean.


