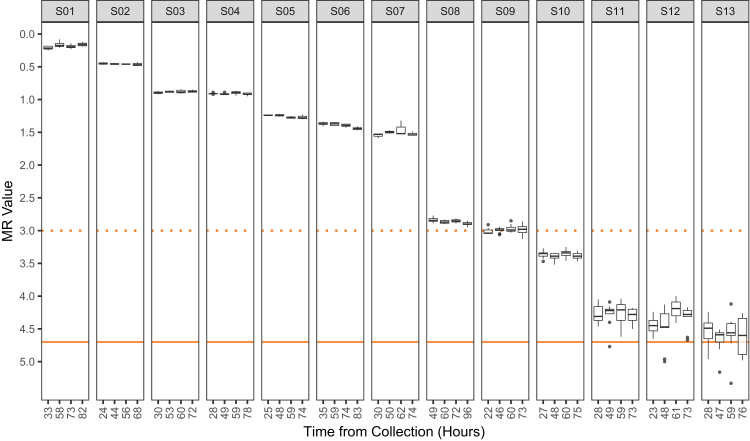
Figure 5.
Whole blood specimen stability. The stability of whole blood RNA for BCR-ABL1 monitoring in the context of the kit was determined by processing aliquots of human blood specimens from chronic myeloid leukemia (CML)–positive patients over an approximate time frame of 72 hours. Each isolate of RNA was tested with the kit a minimum of three times, and in some cases up to nine times (depending on RNA yield). Molecular response (MR) values for each replicate are plotted according to the time they were processed. The median, upper, and lower quartiles are shown by the box-and-whisker plot. Following the recommendations of Tukey,21 the whiskers extend to 1.5 times the upper and lower quantiles (first and third, respectively) and gray points denote values measured outside this range. Actual time point of each specimen's processing is shown rounded to the nearest hour after venipuncture. The dotted orange line is drawn at MR3 (equivalent to 0.1% and major molecular response). The y axis represents measured MR value (with highest BCR-ABL1 analyte level at the top), and the solid orange line is drawn at MR4.7, the limit of detection of the test. Supplemental Table S4 shows estimates of imprecision of ABL1 CT value across post-venipuncture time points for three CML-negative donors.