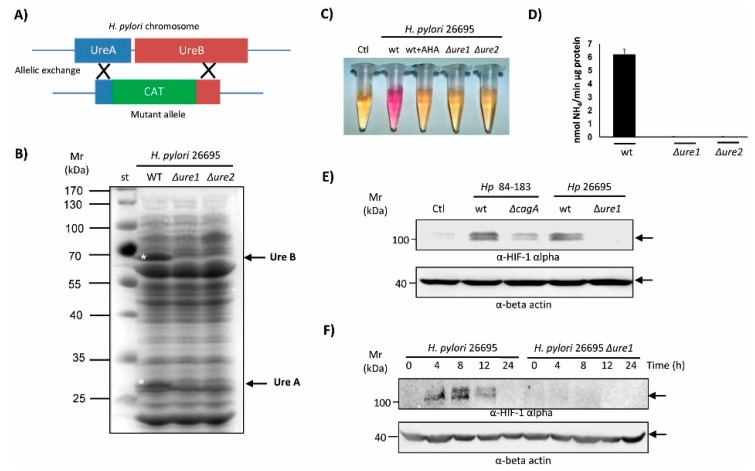
Figure 3.
Characterization of the urease mutant in the H. pylori 26695 strain. (A) Schematic indicating how the urease mutant (ΔureA/B) in the H. pylori 26695 strain was obtained by allelic exchange. (B) Protein extracts (80 µg) from H. pylori 26695 and resistant ΔureA/B clones 1 and 2 were separated by SDS-PAGE (10%) and then stained with Coomassie blue. Asterisks and arrows indicate the positions of UreA and UreB proteins, and the molecular weight markers (10–100 kDa) are shown to the left. A representative gel is shown. (C) A suspension of either parental bacteria or ΔureA/B clones 1 and 2 (Δure1 and Δure2) were added to Brucella broth supplemented with urea 5 mM and the pH indicator red phenol. As a control of the reaction, the urease inhibitor acetohydroxamic acid (AHA, 20 mM) was included. A representative assay is shown. (D) Quantitative determination of urease activity in protein extracts from the H. pylori 26695 parental strain and its ΔureA/B mutants, clones 1 and 2 (Δure1, Δure2). Values are expressed as moles of NH4/min/µg protein (means ± SEM, n = 3, p ≤ 0.05). (E) AGS cells were infected with H. pylori parental 84-183 and 26695 strains, or their ΔcagA and ΔureA/B clon 1 (Δure1) mutants, respectively, at MOI 100 for 8 h. Protein extracts were prepared and separated by SDS-PAGE on 10% gels. HIF-1α and β-actin protein levels were detected by Western blotting. A representative blot is shown. (F) AGS cells were infected for 24 h with H. pylori 26695 or the ΔureA/B mutant clone 1 (Δure1) at MOI 100. Protein extracts were prepared and separated by SDS-PAGE. HIF-1α and β-actin protein levels were detected by Western blotting. A representative blot is shown.