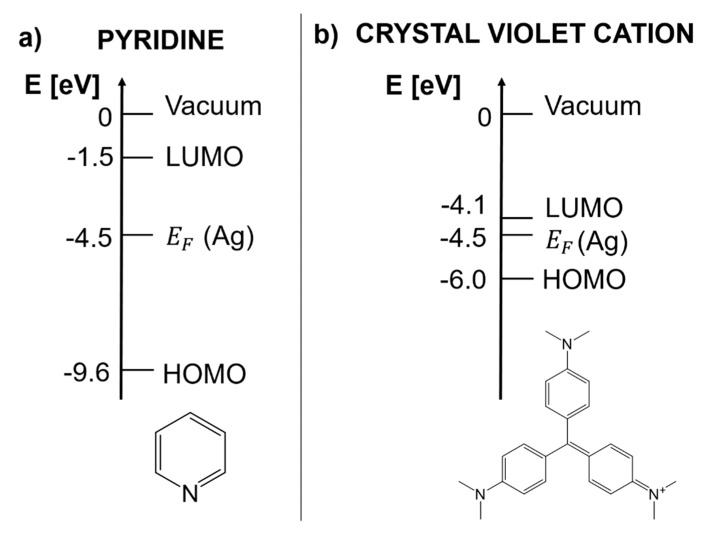
Figure 4.
(a) Energy diagram for pyridine. The highest occupied molecular orbital (HOMO) energy has been approximated as the ionization energy of pyridine, determined from photoelectron spectroscopy measurements [142]; the energy difference between the lowest unoccupied molecular orbital (LUMO) and the Fermi level has been estimated with inverse photoemission spectroscopy [11]; the Fermi level of silver has been estimated by a photoelectric method [9,137,143]. (b) Energy diagram for the crystal violet cation. The HOMO and LUMO energies have been estimated with electrochemical methods [146].