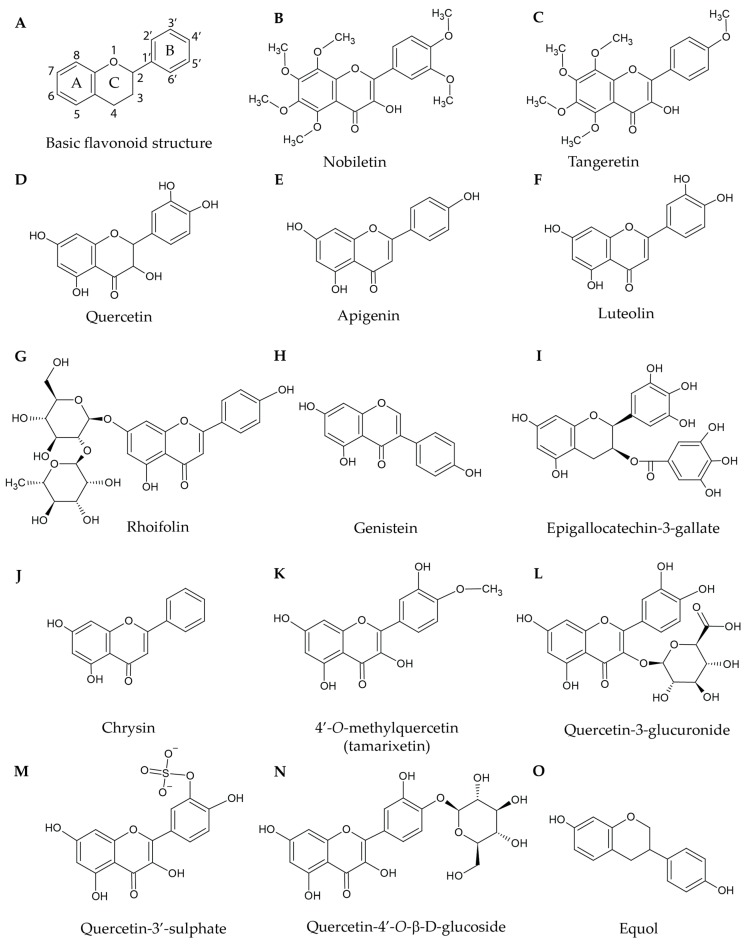
Figure 1.
Structures of selected natural flavonoids and metabolites: (A) The basic structure of flavonoids. A, B, and C denote the A-ring, B-ring, and C-ring respectively. Nobiletin (B) and tangeretin (C) are both heavily methoxylated. Quercetin (D), apigenin (E), and luteolin (F) possess similar structures but differ in the extent of their hydroxylation. Rhoifolin (G) is a flavonoid with a glycoside bond at C7 on the A-ring. Genistein (H) has a simple structure similar to apigenin; however, the B-ring is connected at C3 rather than C2. Epigallocatechin-3-gallate (I) possesses an ester bond connecting the phenyl ring to the flavonoid core. Chrysin (J) is a very simple flavonoid with no hydroxyl groups attached to the phenyl B-ring. 4′-O-methylquercetin (K), quercetin-3-glucuronide (L), and quercetin-3′-sulphate (M) are metabolites of quercetin and are either methylated, glucuronidated, or sulphated, respectively. Quercetin-4′-O-β-d-glucoside (N) has a glucosidic bond at the 4′ position. Equol (O) is a metabolite that is produced by gut bacteria.