Table 1. EC50 Values against Nrf2 Activation of Synthesized Compounds.
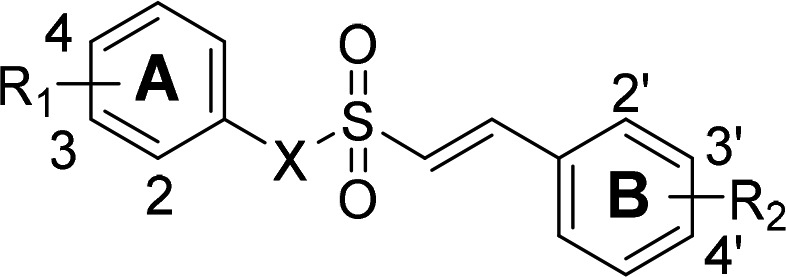
| compd. | X | R1 | R2 | Nrf2 EC50 (μM)a |
|---|---|---|---|---|
| 2a | N | -H | -H | >10 |
| 2b | N | -H | 2′-Cl | >10 |
| 2c | N | 2-OMe | 2′-Cl | 6.350 ± 0.039 |
| 2d | N | 3-OMe | 2′-Cl | >10 |
| 2e | N | 4-OMe | 2′-Cl | >10 |
| 3a | O | -H | -H | 1.040 ± 0.015 |
| 3b | O | 4-OMe | -H | 1.480 ± 0.047 |
| 3c | O | 2-OMe | 2′-Cl | 0.076 ± 0.009 |
| 3d | O | 3-OMe | 2′-Cl | 0.165 ± 0.026 |
| 3e | O | 4-OMe | 2′-Cl | 0.237 ± 0.015 |
| 3f | O | 2-OMe | 3′-Cl | 0.698 ± 0.016 |
| 3g | O | 3-OMe | 3′-Cl | 0.943 ± 0.050 |
| 3h | O | 4-OMe | 3′-Cl | 0.948 ± 0.028 |
| 3i | O | 2-OMe | 4′-Cl | 1.334 ± 0.024 |
| 3j | O | 3-OMe | 4′-Cl | 1.778 ± 0.030 |
| 3k | O | 4-OMe | 4′-Cl | 1.155 ± 0.036 |
| 1b | 2-OMe | 2′-Cl | 0.530 ± 0.025 | |
| SFNc | 0.580 ± 0.024 |
The Nrf2 functional assay was accomplished by a PathHunter U2OS Nrf2 nuclear translocation cell line (93-0821C3, DiscoveRx). U2OS cells were plated at 13,000 cells/well in triplicate with various compound concentrations for 6 h. Nrf2 translocation, which is activation-dependent, was determined using a cell-based functional assay of the mean ± standard error half maximal effective concentration (EC50) values.
Compound 1 developed as an Nrf2 activator in a previous study.24
SFN, sulforaphane: a well-known potent Nrf2 activator.
