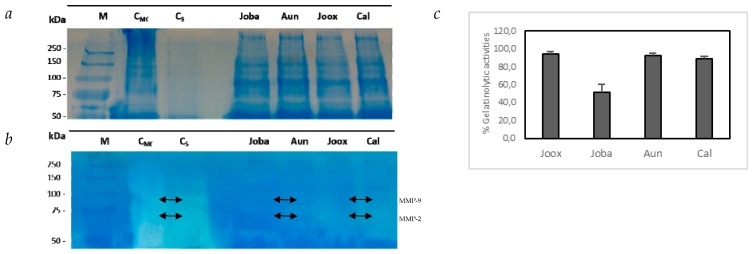
Figure 3.
Gelatinolytic activity for all total protein extracts carried was out on SDS-PAGE NR gel. (a) Control gel on polyacrylamide of 12.5% (m/v), staining by CBB G250, representative of samples from the extracellular medium after incubation with Juniperus oxycedrus subsp. badia (Joba), Arbutus unedo (Aun), Juniperus oxycedrus subsp. oxycedrus (Joox) and Corema album (Cal) extracts: 5 μL from the molecular weight marker (M) and 20 μL extracellular medium were applied of each sample as for medium controls, complete medium control (CMC) and control with saline (CS). (b) Zymographic test carried out on 12.5% (m/v) polyacrylamide gel containing 1% (m/v) gelatin and staining by CBB G250, representative of samples from the extracellular medium, collected at the end of the inhibition test of cell migration. 5 μL of molecular weight marker (M) and 20 μL of all samples were also applied. (c) Proteolytic activity of gelatinases present in the HT29 extracellular media after a 48 h-exposure to 100 μg·mL−1 of buffer-soluble fractions from all species, as quantified by the DQ fluorogenic method. Results are expressed as relative fluorescence as a % of controls and represent an average of at least three replicate experiments ± SD (c). The arrows highlighted the presence of metalloproteinases MMP-9 and MMP-2.