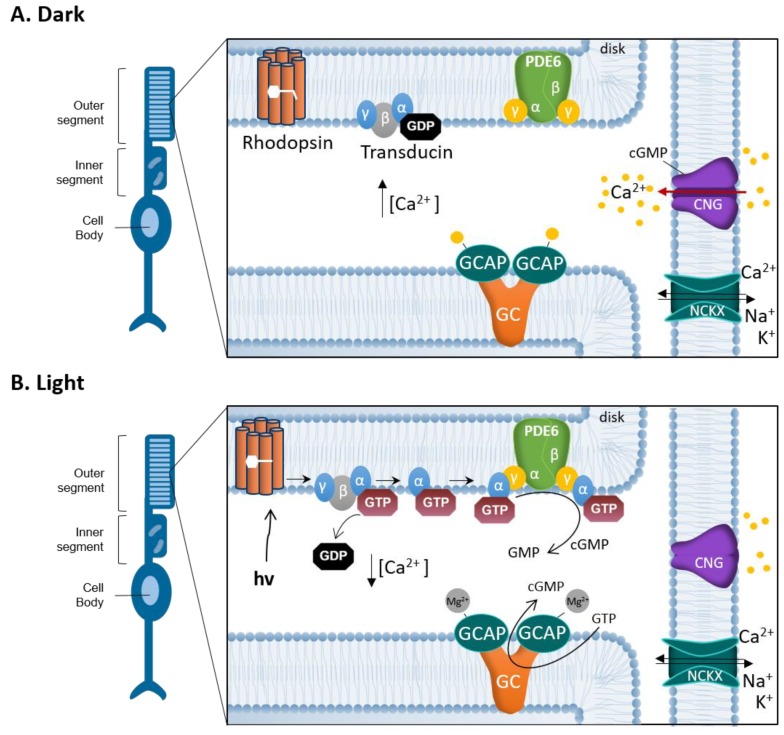
Figure 2.
Phototransduction and the photoreceptor cGMP-Ca2+ feedback loop. Schematic representation of the interplay between cGMP and Ca2+ in the photoreceptor outer segment (OS). (A) In darkness, cGMP binds to the cyclic nucleotide-gated channel (CNGC). The opening of CNGC allows for an influx of Na+ and Ca2+ into the photoreceptor OS. At the same time K+ and Ca2+ ions are constantly extruded via Na+/Ca2+/K+ exchanger (NCKX) creating a continuous influx and outflow of ions called the dark current. Ca2+ binds GC activating proteins (GCAPs), which inhibit the synthesis of cGMP by limiting guanylyl cyclase (GC) activity. (B) In light, photon (hν) absorption induces conformational changes in the rhodopsin protein. Rhodopsin stimulates the GTP-binding protein transducin to detach from heteromeric G-protein complex, by replacing bound GDP with GTP. The activated transducin α subunit binds to the PDE6 complex, abolishing the inhibitory effect exerted by its γ subunits. Activated phosphodiesterase-6 (PDE6) hydrolyses cGMP to GMP, which in turn limits the CNGC opening and leads to a reduction of Ca2+ influx. The closure of the CNGC and a hyperpolarization of the OS, due to continued activity of NCKX, promote the generation of an electro-chemical signal that is transmitted to second order neurons. When OS Ca2+ concentration is reduced, Mg2+ replaces the Ca2+ bound to GCAP, reactivating GCAP and stimulating GC to synthesize cGMP, opening the CNGC again.