Figure 2.
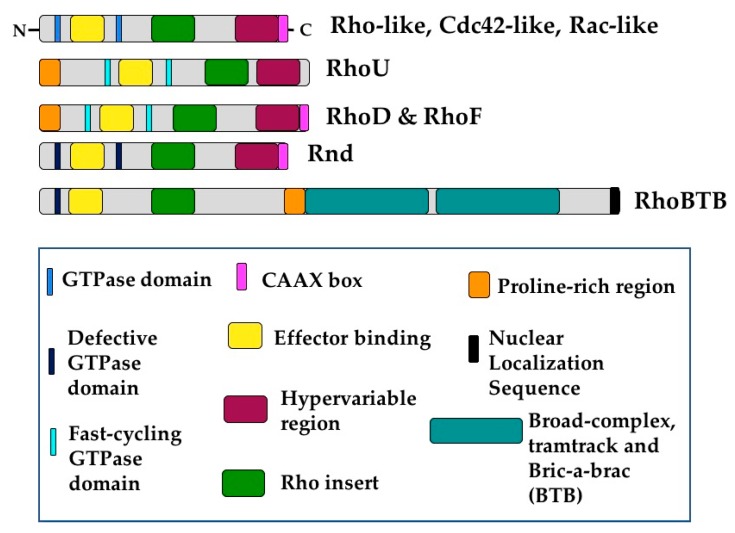
Rho GTPases share conserved structural domains. The essential building blocks of any Rho GTPase are the Rho insert and the GTPase domains. Additionally, Rho GTPases contain effector binding domains and most of them contain C-terminal CAAX domains which can be posttranslationally modified, usually by prenylation. “Classical” Rho GTPases include those of the Rho-like, Rac-like, and Cdc42-like subgroups, as well as RhoU and RhoV, and RhoD and RhoF. RhoU, RhoD, and RhoF deviate from the “classical” formula in that they contain a proline-rich domain at the N-terminus that facilitates binding to other proteins. Additionally, these proteins are considered fast-cycling GTPases, so they are in effect constitutively bound to GTP. Members of the Rnd and RhoBTB subgroups are unable to hydrolyze GTP. Additionally, RhoBTB proteins contain two BTB domains as well as putative nuclear localization sequences.