Abstract
The ubiquitin and hypoxia-inducible factor (HIF) pathways are cellular processes involved in the regulation of a variety of cellular functions. Enzymes called ubiquitin E3 ligases perform protein ubiquitylation. The action of these enzymes can be counteracted by another group of enzymes called deubiquitinases (DUBs), which remove ubiquitin from target proteins. The balanced action of these enzymes allows cells to adapt their protein content to a variety of cellular and environmental stress factors, including hypoxia. While hypoxia appears to be a powerful regulator of the ubiquitylation process, much less is known about the impact of DUBs on the HIF system and hypoxia-regulated DUBs. Moreover, hypoxia and DUBs play crucial roles in many diseases, such as cancer. Hence, DUBs are considered to be promising targets for cancer cell-specific treatment. Here, we review the current knowledge about the role DUBs play in the control of HIFs, the regulation of DUBs by hypoxia, and their implication in cancer progression.
Keywords: Hypoxia, DUBs, E3 ligases, ubiquitylation, HIF, cancer
1. Introduction
Proteins are involved in practically every aspect of cellular functionality. As components of the cytoskeleton, they take part in maintaining structural support: As enzymes they function to catalyze a variety of biochemical reactions, as hormonal and messenger proteins they are involved in signal transduction, and as antibodies they take part in defense reactions. In mammalian cells, the protein turnover rate is rather high: About 30% of newly synthesized proteins are degraded with a half-life of less than 10 min [1]. Therefore, there is no doubt that maintaining protein homeostasis has to be tightly controlled in the cell, as both excessive protein synthesis and degradation can lead to a manifestation of various diseases, including neurodegenerative disorders, viral diseases, and one of the most common leading causes of death worldwide—cancer.
The intracellular degradation of proteins may be achieved in two ways, via lysosomal proteolysis and ubiquitin-mediated proteasomal degradation [2]. Although several lines of evidence have indicated that these two pathways are interlinked, the action of lysosomes is rather broad [2]. In addition to proteins, they also break down various biomolecules from outside and inside the cell (autophagy), including nucleic acids, carbohydrates, and lipids. In contrast to lysosomal degradation, ubiquitin-mediated proteasomal protein degradation is considered to be highly selective and has therefore gained specific interest in cancer therapy. Indeed, the proteasome inhibitors bortezomib, carfilzomib, and ixazomib have been approved for mantle cell lymphoma and multiple myeloma therapy [3,4]. The mechanisms by which proteasomal inhibitors kill cells is rather unspecific and comprises the accumulation of misfolded proteins and reactive oxygen species (ROS), the upregulation of proapoptotic proteins, the stabilization of p53, as well as antiangiogenic actions [3].
Cancers, especially solid cancers, are also characterized by their limited oxygenation (commonly called hypoxia) [5]. To sustain hypoxia, cells initiate an adaptation program where proteins from the hypoxia-inducible transcription factor-α family (HIF) play a key role. Importantly, HIFs themselves are subject to an oxygen-dependent degradation involving ubiquitylation and subsequent proteasomal degradation [6]. Interestingly, the process of ubiquitylation is reversible and carried out by enzymes termed deubiquitinases (DUBs) [7]. While hypoxia appears to be a powerful regulator of the ubiquitylation process, much less is known about hypoxia-regulated DUBs and the impact of DUBs on the HIF system. Therefore, the current review aims to summarize the role DUBs play in hypoxia signaling and cancer progression.
2. Ubiquitin-Mediated Proteasomal Degradation
Ubiquitylation is a highly dynamic process, where ubiquitin (Ub) is covalently linked to lysine residues in target proteins. Although ubiquitylation is best known for its role in marking proteins for subsequent degradation by the 26S proteasome, ubiquitin conjugation has many more diverse functions depending on the nature of the ubiquitin modification (see below). At its most simple level, ubiquitylation requires, apart from the target protein Ub as a tagging factor, the sequential action of a Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzyme [7].
Ubiquitin is a small, highly conserved protein with a molecular weight of 8.5 kDa, which is attached either as a monomer or as an isopeptide-linked polymer to the target proteins. The isopeptide bond between Ub and the target is usually formed between the carboxy terminal glycine residue (G76) of ubiquitin and the ε-amino group of a lysine residue in the target [8].
As mentioned before, the action of three enzymes (E1, E2, and E3) is required for successful attachment of ubiquitin to the substrates [9]. First, the E1 enzyme activates ubiquitin by forming a thioester bond between the carboxy terminal glycine residue of ubiquitin and the active site cysteine in E1. Adenosine triphosphate (ATP) is required for this step. Second, activated Ub is transferred from E1 to the active site of one of 30–40 Ub-conjugating (E2) enzymes. In the third step, Ub is transferred with the help of an E3 Ub ligase from the Ub-loaded E2 to a target substrate that is specifically recognized and bound to E3 [10].
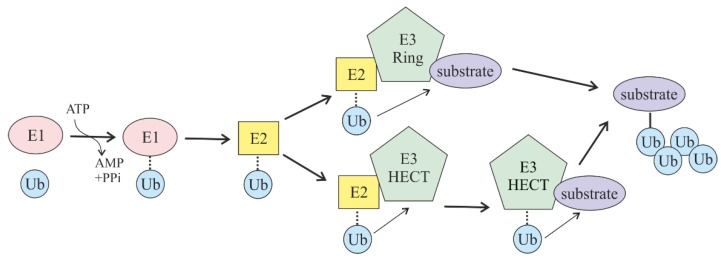
Taking into account that the number of E3 Ub ligases in a cell is ~650, it is of no surprise that these enzymes are acclaimed as the main specificity factors in the whole ubiquitin-mediated proteasomal degradation pathway. There are two main families of E3 Ub ligases, which differ in the mechanism of ubiquitin transfer: Really interesting new gene (RING)-domain-containing and homologous to the E6-AP carboxyl terminus (HECT)-domain-containing ligases. RING E3 ligases (and the structurally related U-box E3s) are complexes that act as a scaffold to ensure the direct transfer of ubiquitin from E2 to the target lysine in the substrate. In contrast, HECT E3 ligases first transfer Ub from E2 to the active-site cysteine within the HECT domain of E3, and from there it is then transferred to a lysine in the substrate bound to E3. Over 92% of the E3 Ub ligases in the cell are RING-domain-containing E3 ligases, while the rest are HECT and other smaller families of ligases (plant homology domain, zinc finger, and U-box) [11] (Figure 1).
Figure 1.
Scheme of protein substrate ubiquitylation. This pathway requires ubiquitin (Ub) and the availability of Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzymes. Two major classes of Ub-ligating enzymes, really interesting new gene (RING)- and homologous to the E6-AP carboxyl terminus (HECT)-domain-containing E3 ligases, are presented.
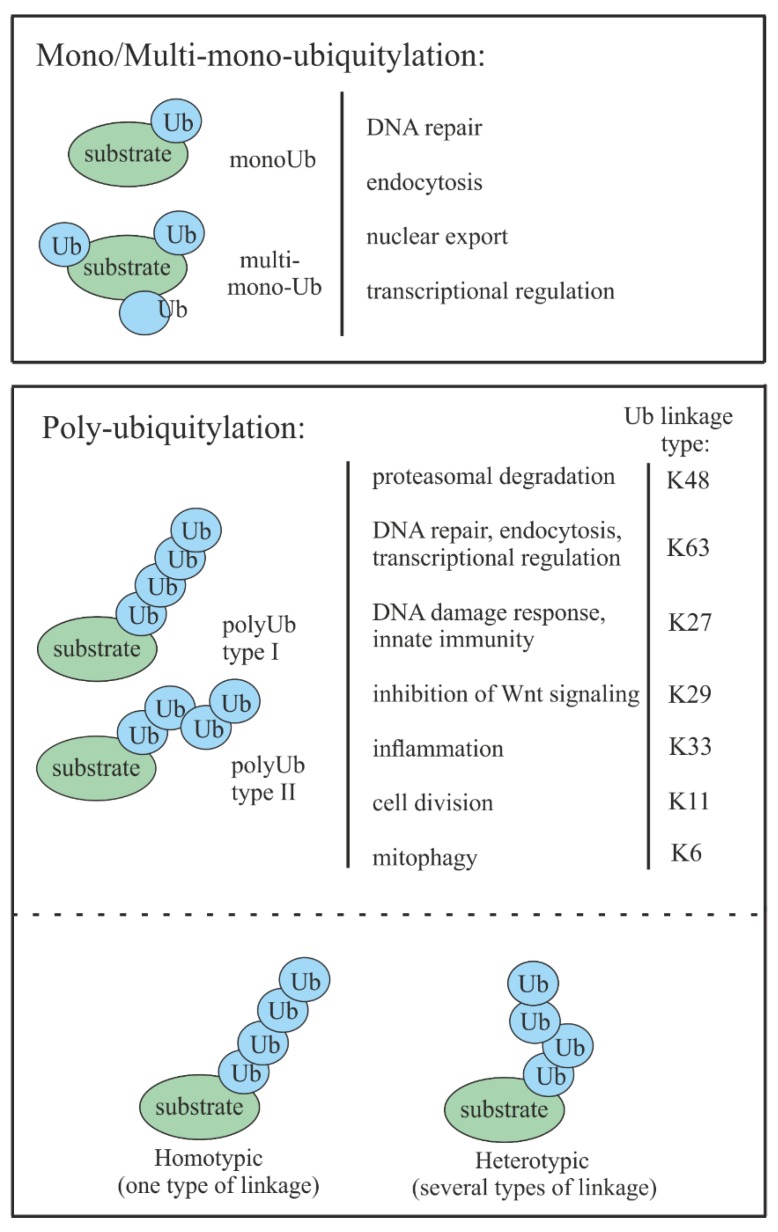
Three different types of ubiquitylations are known: (a) Monoubiquitylation, when only a single molecule of ubiquitin is attached to the target substrate; (b) multi-monoubiquitylation, when several single ubiquitin molecules are attached to the target protein; and (c) polyubiquitylation, when polyubiquitin chains are attached to one or several lysines (K) of the substrate [12]. Monoubiquitylation usually plays the role of a reversible, nonproteolytic signal that is often involved in endocytosis, endosomal sorting, DNA repair, and histone regulation [13,14,15]. Multi-monoubiquitylation is known to be involved in endocytosis and receptor internalization [16,17]. Polyubiquitylation is the most common ubiquitin modification that tags target proteins for degradation, where at least four Ub molecules are required to target substrates to the proteasome [14]. Recently, it has emerged that poly-Ub can have nonproteolytic functions by taking part in DNA repair, transcription regulation, cell division, inflammation, endocytosis, mitophagy, and signaling [18].
In addition to ubiquitylation types, a so-called ubiquitin code exists, which is defined by the specific position of a bond between ubiquitins [19]. It is known that ubiquitin has seven lysine residues, K6, K11, K27, K29, K33, K48, and K63. Each of these lysine residues as well as the primary amine at the N-terminus can be used as a site for the attachment of other ubiquitin molecules. The resulting polymers may contain multiple linkage types in mixed or branched topologies (heterotypic ubiquitin chains) or consist of ubiquitins connected by a single linkage type (homotypic ubiquitin chain). Naturally, the type and position of linkage defines the cell fate of the ubiquitin-tagged proteins [19] (Figure 2).
Figure 2.
Schematic representation of different types of protein substrate Ub modifications together with their roles in cell functioning.
The best-known ubiquitin modification involves K48-linked polyubiquitin chains. This type of modification is commonly found in protein substrates tagged for proteasomal degradation [18]. Although K11-, K29-, and K63-linked chains have also been shown to be involved in proteasomal degradation, they appear to have a more regulatory role in a number of nonproteolytic processes [11], such as DNA repair, transcriptional regulation, or endocytosis [20,21,22]. While the knowledge about K6, K27, K29, and K33 linkages is limited, recent reports have suggested that K6-, K11-, and K33-linked poly- and monoubiquitylations regulate processes such as inflammation, cell division, and mitophagy, respectively [23,24,25].
3. Deubiquitinating Enzymes (DUBs)
The process of ubiquitylation is very dynamic and can be reversed by the action of specialized enzymes known as deubiquitinases (DUBs) [7].
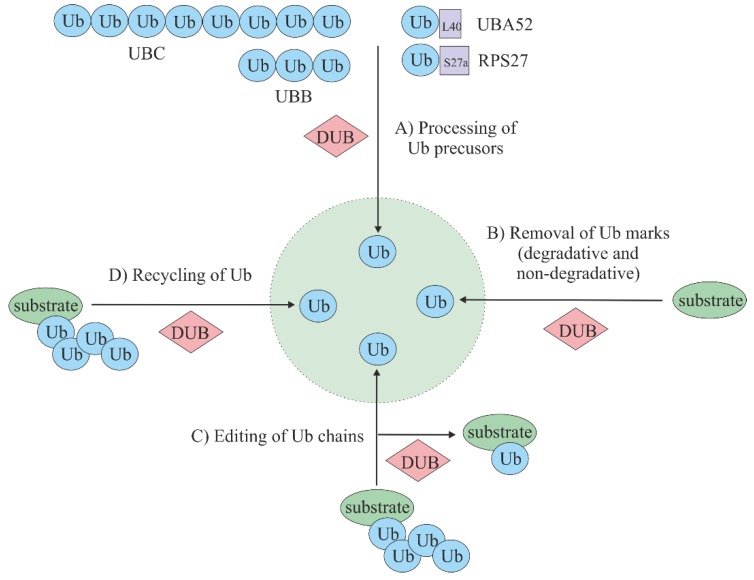
Overall, the general role of DUBs can be presented in four separate pathways that are all linked in order to maintain adequate ubiquitin homeostasis. One of the most important function of DUBs is (a) ubiquitin precursor processing and maturation. In addition, DUBs are capable of (b) removing ubiquitin marks from protein substrates, which can rescue proteins from degradation or modulate signaling. Further important functions of DUBs consist of (c) the editing of ubiquitin chains, which can help to convert one ubiquitin signal type to another; and (d) the recycling of ubiquitin, which ensures that ubiquitin re-enters the ubiquitin pool [26] (Figure 3).
Figure 3.
Cellular role of deubiquitinating enzymes (DUBs). (A) Processing of ubiquitin precursors. Ubiquitin is encoded by four genes: UBA52, RPS27, UBB, and UBC. In the case of UBA52 and RPS27, ubiquitin is produced as a precursor, where a single ubiquitin is attached to ribosomal proteins L40 or S27a. The UBC and UBB genes express precursors comprised of 3–10 single ubiquitins attached “head to tail”. The production of free ubiquitin out of the precursor forms is one of the main roles of DUBs. (B) Removal of degradative or nondegradative marks from protein substrates, therefore rescuing substrates from degradation or modulating ubiquitylation signaling. (C) Editing of ubiquitin chains by changing one type of ubiquitin signal to another (e.g., transformation of polyubiquitin tag to monoubiquitin tag). (D) Recycling that ensures ubiquitin re-enters the ubiquitin pool by preventing ubiquitin degradation in the proteasome or lysosome together with its substrate.
About 100 DUBs are encoded by the human genome, and they are subdivided into six families according to their sequence and structural similarity: JAB1/MPN/MOV34 metallopeptidases (JAMMs), ubiquitin carboxy terminal hydrolases (UCHs), otubain/ovarian tumor proteases (OTUs), Machado–Joseph disease protein domain proteases (MJDs), the newly discovered motif interacting with Ub-containing novel DUB (MINDY) family, and ubiquitin-specific proteases (USPs). The latter class is considered the largest of the DUBs and includes approximately 60 proteases. All of the DUBs are cysteine proteases, except JAMMs, which belong to zinc metalloproteases [27,28]. DUB families that act as cysteine proteases rely on two–three amino acid residues (Cys, His, and optional Asn/Asp) in their active center, ensuring nucleophilic attacks on the isopeptide bond. On the contrary, JAMMs, which coordinate two zinc ions, use the ability to activate H2O in order to attack the isopeptide bond between ubiquitin and the target protein [26,29].
Taking into account emerging reports supporting DUB involvement in many disorders, including cancer, it is not a surprise that DUBs are becoming more and more attractive “druggable” targets [30]. Recent reports have shown that small-molecule DUB inhibitors targeting, e.g., USP14 and UCHL5, could be used as anticancer agents and are being tested in preclinical studies [31,32].
4. Hypoxia and Cancer
Many organisms have evolved adaptive mechanisms for survival under hypoxic conditions [33]. Hypoxia is also a very common stress phenomenon in solid tumors, where, due to increased cell proliferation, large tumor masses are formed. Cancer cells adapt to conditions with low oxygen availability through activating the formation of a tumor vasculature and metabolic reprogramming, including the activation of glycolysis and inhibition of mitochondrial function [5,34]. The activation of HIFs is usually the key event in this adaptation to low oxygen concentrations. HIFs exist as heterodimers composed of the hypoxia-inducible α-subunit (HIF-α) and the constitutively expressed β-subunit, also known as the arylhydrocarbon receptor nuclear translocator (ARNT) [35]. So far, three major members of the HIF α-family have been described: HIF-1α, HIF-2α, and HIF-3α [36,37,38]. From the three known HIF α-family members, HIF-1α is the best characterized. Initially, it was identified due to its sensitivity to low O2 levels, but it is now clear that HIF-1α can also be regulated by other factors, such as the activation or loss of tumor oncogenes or suppressors. There is evidence that has indicated HIF-1α accumulation even under normoxic conditions in tumor cells after the loss of tumor suppressors such as von Hippel-Lindau (VHL) or Phosphatase and Tensin homolog (PTEN) or the activation of oncogenes such as Rat sarcoma (RAS), sarcoma (SRC), and phosphoinositide 3-kinase (PI3K) [34,39].
HIF-1α is known to regulate the expression of more than 300 genes and noncoding RNAs [6,40], which control genomic stability, drug resistance, angiogenesis, vascular tone, glucose metabolism, cell proliferation, and survival. As a result, elevated levels of HIF-1α are often associated with a poor prognosis for cancer patients [41,42].
Although there have been emerging reports about the role of hypoxia, HIFs, and DUBs in cancer development, there is still a lack of knowledge about their reciprocal regulation.
5. Degradation of HIFs
An abundance of HIF α-subunits is mainly regulated on the post-translational level via its degradation [43,44,45,46,47], although mechanisms involving HIF transcription [34] and HIF-1α mRNA translation have also been described [35].
The stability of HIF α-subunits is heavily reduced under normoxic conditions due to its rapid degradation via the proteasomal pathway [36], though lysosomal degradation can contribute to HIF-1α degradation during chaperone-mediated autophagy (CMA) [37]. In addition to hypoxic conditions, several signaling pathways have been shown to regulate the stability and transactivity of HIF-1α via post-translational modifications, including hydroxylation, acetylation, phosphorylation, SUMOylation, and ubiquitylation [38]. Therefore, it is not a surprise that several E3 ligases and DUBs can be involved in HIF α-subunit regulation (Figure 4).
Figure 4.
E3 Ub-ligating and deubiquitinating enzymes (DUBs) involved in hypoxia-inducible factor (HIF)-α stability and signaling. DUBs and E3 Ub ligases involved in the oxygen-dependent regulation of HIF-1α and HIF-2α degradation are depicted in red. DUBs and ubiquitin E3 ligases involved in oxygen-independent regulation of HIF degradation are depicted in black. DUBs involved in the regulation of HIF mRNA stability and/or expression are depicted in blue. Non-enzymatic and/or indirect regulations are indicated in italics.
5.1. Oxygen-Dependent Degradation of HIFs
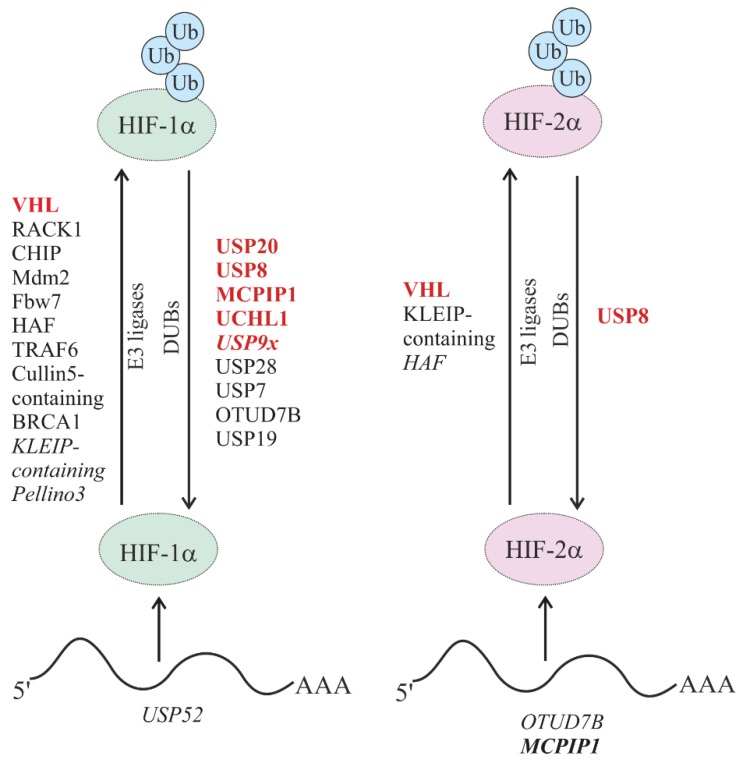
The protein stability of HIF-α is crucially regulated by the oxygen-dependent hydroxylation of two proline residues (P402 and P564 in human HIF-1α; P405 and P531 in human HIF-2α) located in the oxygen-dependent degradation domain (ODDD). The hydroxylation reactions are carried out by at least three HIF proline 4-hydroxylases known as proline hydroxylase domain-containing enzyme family members (PHD1–3) or egl-9 (Caenorhabditis elegans) gene Homologs (EGLN1-3), which function as cellular O2 sensors [48]. The hydroxylation of the proline residues marks the HIF α-subunits for ubiquitylation through an E3 ligase complex containing the tumor suppressor protein von Hippel-Lindau (VHL) and for subsequent proteasomal degradation [49]. Moreover, another hydroxylation at an asparagine residue in the C-TAD of HIF α-subunits carried out by the factor-inhibiting HIF (FIH) alters their transactivity by preventing recruitment of the coactivator p300 [50]. Interestingly, VHL itself is subject to proteasomal degradation promoted by an E3 ligase, SMURF1, which is deubiquitylated by the DUB USP9x [51]. Several E3 ligases and DUBs have been reported to participate in the regulation of HIF-α degradation in an oxygen-dependent manner (Figure 5) (Table 1).
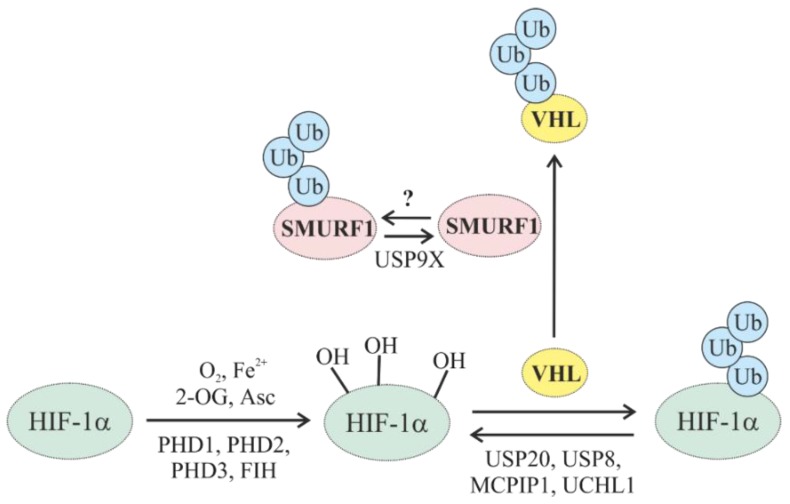
Figure 5.
Involvement of E3 ligases and DUBs in the oxygen-dependent regulation of HIF. HIF-α subunits are hydroxylated in an O2-, Fe2+-, 2-oxoglutarate (2-OG)-, and ascorbate-dependent reaction. Hydroxylated HIF-α subunits are then recognized by von Hippel-Lindau (VHL), ubiquitylated, and degraded by the proteasome. VHL itself can be degraded by the E3 ligase SMURF1 (SMAD ubiquitination regulatory factor-1), which can be opposed by USP9X. VHL-mediated ubiquitylation can be antagonized by the DUBs USP20, USP8, MCPIP1, and UCHL1. E3 ligases are depicted in bold.
Table 1.
E3 ligases and DUBs affecting the oxygen-dependent regulation of HIFs and their involvement in cancer.
| Enzymes | Involvement in HIF Regulation | Involvement in Cancer | References |
|---|---|---|---|
| Ub E3 Ligases | |||
| VHL-containing | Ubiquitylates hydroxylated HIF-α for proteasomal degradation |
|
[53,54,55,147] |
| SIAH 1/2 | Ubiquitylate PHD1/3, leading to HIF-1α stabilization; SIAH1 facilitates FIH degradation via the proteasomal pathway |
|
[60,148,149,150,151,152,153] |
| SPOP | Ubiquitylates PHD1, promoting its proteasomal degradation |
|
[61,154,155,156] |
| DUBs | |||
| USP8 | Reverses the VHL-mediated degradation of HIF-1α and HIF-2α |
|
[72,74,135,157,158] |
| USP9X | Affects the ubiquitylation of HIF-1α indirectly by reducing VHL via deubiquitylation of the E3 ligase SMURF1, which targets VHL |
|
[51,159,160,161,162] |
| USP20 | Counteracts the VHL-mediated ubiquitylation of HIF-1α |
|
[63,65,66,67] |
| MCPIP1 | Deubiquitylates HIF-1α; suppresses the levels of HIF-1α and SIRT-1 miR repressors |
|
[68,69,70,71,163] |
| UCHL1 | Abrogates VHL-mediated ubiquitylation of HIF-1α |
|
[79,82,83,84,164,165] |
5.1.1. Oxygen-Dependent Degradation of HIF by E3 Ub Ligases
So far, the tumor suppressor protein VHL is the major E3 ligase substrate recognition component in oxygen-dependent HIF-α degradation. In addition to VHL, the RING–E3 ligase complex also contains Ring Box 1 (RBX1), Cullin 2, and Elongin B and C [52]. Often, VHL is mutated in hemangioblastomas [53], pheochromocytomas [54], and clear-cell renal carcinomas [55], illustrating the importance of VHL in the pathogenesis of these diseases. Tumors lacking VHL are in general characterized by increased HIF-α levels and the expression of HIF target genes: Vascular endothelial growth factor (VEGF) and erythropoietin (EPO) [56,57]. These characteristics validate the correlation between HIF-α and VHL [49].
5.1.2. Regulation of HIF Hydroxylases (PHDs and FIH) through Ubiquitylation
Even though HIF hydroxylase activity is mainly regulated via O2 availability, their abundance is also regulated by various E3 ligases catalyzing their ubiquitylation and consequent proteasomal degradation.
Although there are so far no known E3 ligases regulating PHD2, the protein stability and half-life of PHD2 are reported to be downregulated by its interactor peptidyl prolyl cis/trans isomerase FK506-binding protein 38 (FKBP38) [58].
The stability and abundance of PHD1 and PHD3 in the cell are regulated by the seven in absentia homolog (SIAH1/2) E3 ligases. Their activity increases under hypoxic conditions, resulting in less availability of PHD1/3 and HIF-1α stabilization [59]. The E3 ligase SIAH1 has also been reported to be a binding partner of the asparaginyl hydroxylase FIH, which is known as an oxygen-dependent repressor of HIF-1α, facilitating FIH degradation via the ubiquitin–proteasome pathway under hypoxic conditions [60]. Thus, SIAH ubiquitin ligases play a role as regulators of both types of HIF hydroxylases (PHDs and FIH) and represent an important part of feedback regulation.
In addition to SIAH1/2, the Cullin 3-based E3 ligase speckle-type POZ protein (SPOP) has been shown to regulate PHD1 via polyubiquitylation and proteasomal degradation. In line with this, it has been reported that elevated PHD1 levels and a loss of SPOP were linked to prostate cancer growth [61].
Interestingly, a reciprocal regulation of E3 ligases by HIF hydroxylases has also been reported. Thereby, the ankyrin repeat and SOCS box protein 4 (ASB4), which is highly expressed in vascular tissue and serves as the substrate-recognizing protein of the SCF-like Elongin-Cullin-SOCS-box E3 ubiquitin–protein ligase complex, was shown to be hydroxylated by FIH, leading to vascular differentiation in an oxygen-dependent manner [62]. Together, the O2-dependent abundance of HIF-α transcription factors and their major hydroxylases is regulated by feedback cycles involving several E3 ligases (Figure 6).
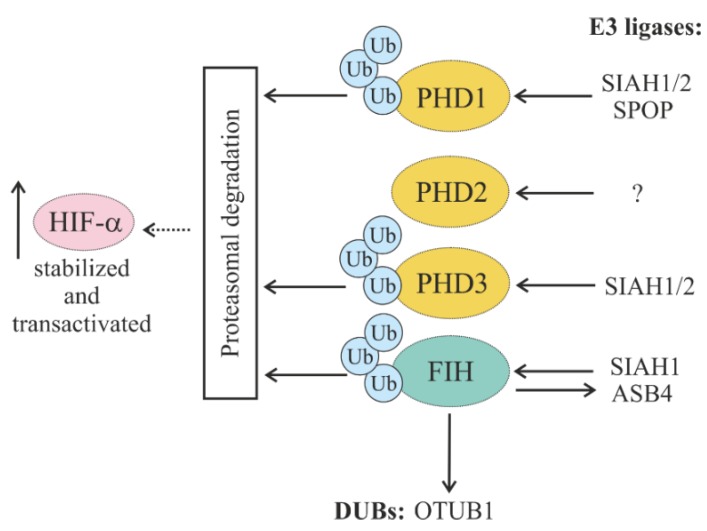
Figure 6.
Schematic representation of HIF prolyl hydroxylases (PHDs) and factor-inhibiting HIF (FIH)) interplaying with E3 ligases and DUBs and their impact on HIF cellular abundance. Seven in absentia homologs ½ (SIAH1/2) and speckle-type POZ protein (SPOP) promote ubiquitylation of PHD1 and PHD3. So far, no E3 ligase is known for PHD2. Ankyrin repeat and SOCS box protein 4 (ASB4) and Otubain-1 (OTUB1) are hydroxylated by FIH.
5.1.3. Regulation of Oxygen-Dependent HIF Degradation by DUBs
In the context of the oxygen-dependent HIF degradation pathway, there are only a few DUBs that appear to regulate, mainly HIF-1α.
The first described DUB that was shown to counteract VHL-mediated ubiquitylation of HIF-1α was USP20 (VDU2) [63]. According to those findings, USP20 binds to and stabilizes HIF-1α. As a consequence of this interaction, USP20 induces the expression of HIF-1α target genes, such as vascular endothelial growth factor (VEGF). On the other hand, the VHL-interacting deubiquitinase USP33, which has a strong homology with USP20 in its N-terminus and C-terminus and shares an approximately 59% identity with USP20, is not able to bind and stabilize HIF-1α [63]. Besides its involvement in hypoxia signaling, USP20 is also reported to be dysregulated in cancer, and its depletion is known to be associated with increased chromosomal aberrations, malignant transformation, and tumor growth [64]. Due to its ability to inhibit the malignant characteristics of gastric cancer cells via the positive regulation of claspin, USP20 is considered to play a tumor-suppressing role in gastric cancer [65]. Further, it has been shown that claspin expression in gastric cancer samples correlates with USP20 expression and that low claspin and USP20 levels are associated with worse overall survival [66]. However, these findings were contradicted by a recent report showing that USP20 regulates the deubiquitylation of β-catenin to control its stability, thereby inducing proliferation, invasion, migration, and chemoresistance in multiple cancer cells [67]. Thus, it remains open whether USP20 is a tumor suppressor or activator: Cell type-dependent effects are likely to be considered.
HIF-1α can also be deubiquitylated by the monocyte chemoattractant protein-1 (MCP-1)-induced protein-1 (MCPIP1). MCPIP1 is a zinc-finger protein with ribonuclease activity that is mainly crucial in the regulation of stability of transcripts related to inflammatory processes. This process appears to be rather important in vessel growth, as MCPIP causes HIF-1α deubiquitylation and nuclear localization and the induction of its target genes, such as Cyclooxygenase-2 (COX-2) and VEGF [68]. The MCPIP1–HIF axis also appears to be important in the protection of the liver against ischemia reperfusion injury [69] and the development of clear-cell renal carcinoma [70]. Moreover, the antidicer RNase activity of MCPIP is able to suppress the levels of miRs modulating HIF-1α and sirtuin-1 (SIRT-1) expression, which also contributes to angiogenesis activation [68].
MCPIP1 is also shown to affect HIF-2α at the transcript level, and HIF-2α in turn also regulates the expression of MCPIP1 [70]. Further, it has been demonstrated that MCPIP1 can function as a tumor suppressor, as it is able to induce the apoptosis of breast tumor cells by selectively enhancing the decay of mRNA necessary for the expression of antiapoptotic genes (Bcl2L1, Bcl2A1, RelB, Birc3, and Bcl3) [71]. Moreover, it has been found that MCPIP1 depletion increases cancer cell proliferation [70].
The deubiquitinase USP8 has been found to be another protein that can reverse the VHL-mediated degradation of HIF-1. The action of USP8 involves binding to the PER-ARNT-SIM (PAS) domain of HIF-1α and is linked to the maintenance of a basal HIF-1α level under normoxia, which is essential for rabaptin-5 expression and endosome trafficking-mediated ciliogenesis. Further, it was shown that USP8 also likely functions as a DUB for HIF-2α [72]. Apart from HIFs, USP8 is involved in epidermal growth factor receptor (EGFR) turnover, thus rescuing EGFR from lysosomal degradation [73], and mutations in the USP8 gene have been found in corticotroph adenomas, which could cause Cushing’s disease via activation of EGFR signaling [74].
The X-linked deubiquitinase USP9x was reported to affect the ubiquitylation status of HIF-1α indirectly by reducing VHL protein levels via the deubiquitylation of SMURF1, an E3 ligase targeting VHL [51]. USP9x, due to its ability to regulate SMAD family member 4 (SMAD4) and apoptosis signal-regulating kinase 1 (ASK1), is also involved in regulating cancer-associated transforming growth factor- β (TGF-β) [75] and mitogen-activated protein kinase (MAPK) signaling pathways [76]. Decreased levels of both USP9x mRNA and protein were reported to correlate with poor survival in patients with pancreatic ductal tumors, supporting its role as a tumor suppressor [77]. In addition, a correlation between the level of USP9x and the pro-survival-induced myeloid leukemia cell differentiation protein (MCL-1) was shown in follicular lymphomas and diffuse large B-cell lymphomas [78].
Ubiquitin carboxyl terminal hydrolase L1 (UCHL1) could abrogate VHL-mediated ubiquitylation of HIF-1α and promote metastasis in murine models of pulmonary metastasis [79]. Moreover, recent findings have shown that UCHL1 is subjected to oxidative carbonylation, which hampers its activity [80,81] and links its function to oxygen signaling. In addition, the levels of UCHL1 were shown to correlate with HIF-1α levels and to associate with a poor prognosis in patients with breast and lung cancer [79]. UCHL1 was also reported to be overexpressed in gastric cancer [82] and in myelomas [83], while it was silenced via methylation in several colon cancer cell lines [84], illustrating its potentially dual role in cancer development. Further, UCHL1-mediated HIF-1 dependence changed the antioxidant cellular status by increasing the intracellular glutathione levels, which promoted conversion of the cells into a radioresistant phenotype [85]. Interestingly, UCHL1 not only functions as a DUB, but in vitro, upon the formation of dimers, it was shown to act as a ubiquitin ligase [86].
Together, O2-dependent HIF degradation is regulated by a complex network of DUBs and E3 ligases that are on different levels of control and directly affect HIF ubiquitylation or indirectly affect HIF hydroxylases.
5.2. Oxygen-Independent Regulation of HIFs
Although the O2-dependent and VHL-mediated degradation system is the predominant one regulating HIF-α subunit stability, there are other O2-independent mechanisms that modulate their stability (Figure 7) (Table 2).
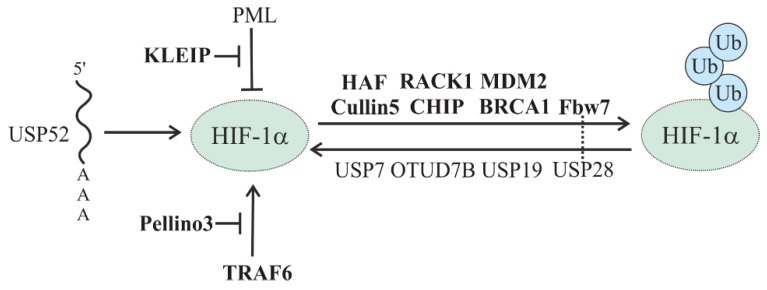
Figure 7.
Involvement of E3 ligases and DUBs in the oxygen-independent regulation of HIF. E3 ligases are depicted in bold. The dotted line indicates known direct interactions between an E3 ligase and DUB.
Table 2.
E3 ligases and DUBs affecting the oxygen-independent regulation of HIFs and their involvement in cancer.
| Enzymes | Involvement in HIF Regulation | Involvement in Cancer | References |
|---|---|---|---|
| Ub E3 Ligases | |||
| RACK1 | Competes with HSP90 for binding to HIF-1α to drive HIF-1α degradation |
|
[87,166,167,168,169,170,171,172,173] |
| CHIP | Promotes HIF-1α but not HIF-2α degradation via both proteasomal or lysosomal machinery |
|
[46,94,95,96,97,174,175,176,177,178,179,180,181] |
| MDM2 | Regulates HIF-1α stability directly due to E3 ligase activity or indirectly by forming a ternary complex, which is degraded in a p53-dependent manner |
|
[98,99,182,183,184] |
| Fbw7 | Recruited to GSK-3-phosphorylated HIF-1α for proteasomal degradation. |
|
[110,111,112,185,186,187,188] |
| HAF | Selectively degrades HIF-1α and promotes HIF-2α transactivation during hypoxia |
|
[101,102,189,190,191] |
| TRAF6 | Increases HIF-1α polylysine-63 ubiquitylation, protecting it from proteasomal degradation; TRAF6-ATM-H2AX signaling axis promotes HIF1α stabilization and activation |
|
[104,192,193,194,195,196] |
| Cullin 5-containing | Involved in HSP90-mediated regulation of HIF-1α stability |
|
[88,197,198,199,200,201] |
| KLEIP-containing | By reducing levels of PML, leads to a activation of mTOR, which promotes HIF-1α signaling; stabilizes mRNA expression of HIF-2α |
|
[107,108] |
| BRCA1 | Regulates stability of HIF-1α (NRM) |
|
[109,202,203] |
| Pellino3 | Ubiquitylates TRAF6 with polylysine-63 to block its interaction with HIF-1α, making it more prone to proteasomal degradation | [106] | |
| DUBs | |||
| USP7 | Deubiquitylation of HIF-1α |
|
[119,204,205,206] |
| USP19 | Stabilizes HIF-1α and interacts with SIAH1/2 and PHD1/3 regulators |
|
[121,122,207,208] |
| USP28 | Counteracts Fbw7-mediated HIF-1α ubiquitylation |
|
[111,114,115,116,117] |
| USP52 | Stabilizes HIF-1α mRNA |
|
[124,209] |
| OTUD7B | Stabilizes HIF-1α and E2F1 transcription factor to control the expression of HIF-2α mRNA; affects HIF-2α at the transcript level |
|
[70,125,127,210,211,212,213] |
5.2.1. HSP-Mediated HIF Degradation
One of the first proteins involved in O2-independent HIF degradation was the receptor for activated C kinase 1 (RACK1). RACK1, in concert with Elongin-C/B, was found to compete with heat shock protein 90 (HSP90) for binding to the HIF-1α PAS-A domain and to recruit Elongin-C/B and other components of the VHL E3 ubiquitin ligase complex to drive HIF-1α degradation [87]. In addition, the RING E3 ubiquitin ligase scaffold protein Cullin 5 was also shown to be involved in HSP90-mediated regulation of HIF-1α stability [88].
Further, the action of RACK1 could be inhibited by the calcium- and calmodulin-dependent serine/threonine phosphatase calcineurin. Thereby, the catalytic domain of calcineurin binds to RACK1 and dephosphorylates serine 146, which inhibits RACK1 dimerization and the recruitment of Elongin-C [89]. The mammalian septin family member SEPT9v1, a specific interactor of HIF-1α but not HIF-2α, was reported to negatively regulate this mechanism by preventing HIF-1α interaction with RACK1 [90]. A second negative regulator is the cleaved intracellular domain of the receptor-tyrosine kinase ERBB4, which also interacts with HIF-1α and inhibits RACK1-dependent HIF-1α degradation [91].
RACK1 complex-mediated HIF degradation can also be modulated by spermidine/spermine N-acetyltransferasees-1 and -2 (SSAT1, -2). While SSAT1 acts by stabilizing the interaction between HIF-1α and RACK1, SSAT2 stabilizes the interaction between VHL and Elongin-C. Thus, the paralogs SSAT1 and SSAT2 can play complementary roles in promoting HIF-1α degradation [92,93].
Heat shock protein 70 (HSP70), through recruiting the ubiquitin ligase C terminus of the HSC70-interacting protein (CHIP), promotes HIF-1α but not HIF-2α degradation via both proteasomal and autophagic machinery. The disruption of HSP70–CHIP interaction blocks HIF-1α degradation mediated by HSP70 and CHIP [46,94,95]. Moreover, CHIP was shown to be required for the chaperone-mediated degradation of HIF-1α by the lysosome. Thereby, the pentapeptide region of HIF-1α between N529 and L533 was reported to bind heat shock 70-kDa protein 8 (HSPA8) and lysosome-associated membrane protein 2A (LAMP2A), which recruited the K63-ubiquitylated HIF-1α to the lysosome [96,97].
5.2.2. Other E3 Ligases in the Oxygen-Independent Degradation of HIF
Mouse double-minute 2 homolog proto-oncogene (MDM2) was reported to be implicated in HIF-1α regulation via both a direct [98] and p53-dependent mechanism [99]. In the case of the direct action, it was shown that MDM2 could regulate HIF-1α stability due to MDM2’s E3 ligase activity. Moreover, the action of MDM2 under hypoxia was controlled by the PTEN/PI3K/AKT signaling axis [98]. In the indirect mechanism, MDM2 was found to form a ternary p53/MDM2/HIF-1α complex, which is degraded in a p53-dependent manner. Similarly to p53, another tumor suppressor, TAp73, is thought to function as a scaffold that mediates the association between MDM2 and HIF-1α and its oxygen-independent proteasomal degradation [100].
Hypoxia-associated factor (HAF), which acts as a ubiquitin E3 ligase, is reported to play a dual role in the regulation of HIF-α stability. Thereby, it is supposed to mediate a switch from HIF-1α to HIF-2α-dependent transcription during tumor hypoxia by selectively degrading HIF-1α and promoting HIF-2α transactivation without affecting HIF-2α levels [101,102]. Additionally, the ability of HAF to activate HIF-2α-dependent transcription is dependent on hypoxia-induced HAF SUMOylation [103], thus adding another layer of complexity here.
In contrast to the view that the ubiquitylation of HIF is commonly associated with its degradation, the E3 ligase TNF receptor associated factor 6 (TRAF6) was reported to increase HIF-1α polylysine-63 ubiquitylation and to protect it from proteasomal degradation [104]. It was also reported that TRAF6-mediated monoubiquitylation and ATM-mediated phosphorylation of histone H2AX, which interacts with HIF-1α to prevent its degradation, promoted HIF-1α-driven tumorigenesis, glycolysis, and metastasis [105]. Interestingly, TRAF6-mediated HIF-1α stabilization can be switched off by the E3 Ub ligase Pellino3. Thereby, Pellino3 ubiquitylates TRAF6 with lysine 63-linked polyubiquitin chains to block its interaction with HIF-1α, resulting in reduced lysine 63-linked polyubiquitylation of HIF-1α, making it more prone to lysine 48-linked polyubiquitylation and proteasomal degradation [106].
Another Ub E3 ligase component having a positive effect on HIF is the BTB-kelch protein (KLEIP, also known as KLHL20). This HIF-1-induced protein functions as a substrate adaptor of Cullin 3-based ubiquitin ligases. Together with cyclin-dependent kinase 1/2 (CDK1/2) and peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), KLEIP mediates proteasomal degradation of the tumor suppressor promyelocytic leukemia protein (PML). The reduced PML levels lead to a derepression of mammalian target of rapamycin (mTOR), which in turn participates in a feedback mechanism to amplify HIF-1α signaling, thus facilitating tumor progression [107]. Moreover, stabilization and mRNA expression of HIF-2α were found to be regulated by KLEIP. Despite the molecular details of KLEIP-dependent HIF-2α regulation being largely unclear, they appear not to be involved in cancer, but rather in the regulation of late-stage pulmonary maturation in mice [108].
An interaction between the tumor suppressor breast cancer type 1 susceptibility protein (BRCA1), which possesses ubiquitin E3 ligase activity, and HIF-1α was also found to be important in regulating HIF-1α stability in human breast cancer cells. However, so far it is unclear whether BRCA1-dependent ubiquitylation is involved in this process [109].
It is now well known that the phosphorylation of HIF-1α by glycogen synthase kinase-3 (GSK-3) is the signal for the recruitment of the E3 ubiquitin ligase F-box protein Fbw7. Similarly to VHL, the Fbw7-containing E3 ubiquitin ligase tags HIF-α subunits with ubiquitin, therefore promoting their proteasomal degradation [110,111,112].
5.2.3. Oxygen-Independent Regulation of HIF Stability by DUBs
The above-mentioned Fbw7-mediated process of HIF-1α ubiquitylation is reversible and can be antagonized by USP28 [111]. This process affects hypoxia- and HIF-1α-dependent cell proliferation, colony formation, and angiogenesis [111]. Although the USP28-dependent regulation of HIFα is linked with tumorigenesis, USP28 is also known to stabilize oncoproteins such as c-MYC [113], c-JUN, and NOTCH [114]. Hence, the role of USP28 in tumorigenesis may vary depending on the tissues or cells involved. While reports have indicated that a lack of USP28 could reduce colon cancer in mice [114] and that USP28 could also be a prognostic marker in bladder [115] and gastric cancers [116], a lack of USP28 promoted liver cancer and correlated with a worse survival of patients with invasive ductal breast carcinoma: Mouse xenograft experiments with USP28-lacking breast cancer cells supported these findings [117].
USP7 (HAUSP), which is known to regulate p53 availability in the cell through its direct stabilization and/or indirectly by stabilizing MDM2 [118], was shown to have the ability to deubiquitylate HIF-1α under normoxia and to promote epithelial–mesenchymal transition and carcinogenesis [119]. Interestingly, hypoxia enhanced the function of USP7 via K63-linked polyubiquitylation at K443, the latter mediated by the E3 ubiquitin ligase HectH9 [119]. In addition, hypoxia-dependent USP7 induction was found to be associated with histone 3 lysine 56 (H3K56) acetylations that were mediated by the histone acetylase CREB-binding protein (CBP) [119].
Another DUB, USP19, which can affect the degradation of ER-associated degradation (ERAD) substrates [120], has also been proposed to function as a regulator in the hypoxia signaling pathway. This was based on findings showing that USP19 can interact with the bHLH-PAS domain of HIF-1α, but not HIF-2α, and with SIAH1 and SIAH2, which are known components of the hypoxia pathway since they regulate the presence of PHD 1/3 enzymes [121,122]. In the absence of USP19, HeLa cells fail to mount an appropriate response to hypoxia, indicating an important role of this enzyme in both normal and pathological conditions [123].
USP52 is another important regulator of HIF-1α, but it is active toward HIF-1α mRNA. This pseudo-DUB/deadenylase appeared to be a key component of the P-bodies required to prevent HIF-1α but not HIF-2α mRNA destabilization. Thereby, depletion of USP52 reduced the HIF-1α mRNA levels via a 3’-untranslated region-dependent but poly(A)-tail-length-independent interference mechanism, with the effect that HIF-1α-regulated hypoxic targets were expressed at a lower level [124].
The DUB OTU domain-containing protein 7B (OTUD7B or Cezanne) was reported to be of regulative importance and to protect HIF-1α and HIF-2α [125]. The regulation of OTUD7B expression in response to hypoxia itself seemed to be selective, with endothelial cells showing an induction of OTUD7B expression [126] and no OTUD7B response in HeLa or U2OS cells [125]. However, the knockdown of OTUD7B decreased HIF-1α and HIF-2α levels and increased K11 linkages on HIF-1α, but not K48 linkages. Further, the effects of OTUD7B on HIF-1α were not dependent on PHD-mediated HIF-1α hydroxylation, but required VHL. Interestingly, experiments interfering with both the function of the proteasome and lysosomal HIF-1α degradation have indicated that OTUD7B effects were mediated by chaperone-mediated lysosomal HIF-1α degradation [125]. Moreover, HIF-2α appeared to be differently regulated, since VHL inactivation was unable to rescue the OTUD7B-mediated reduction of HIF-2α. Indeed, OTUD7B was found to control the expression of HIF-2α mRNA via the transcription factor E2F1 binding to two sites in the HIF2A promoter. Moreover, OTUD7B was found to regulate E2F1 protein abundance and hence take part in maintaining its basal levels [127]. Overall, these data suggest that mitogenic signals can promote cell cycle progression, in particular under hypoxia, in an OTUD7B- and transcription factor E2F1-dependent manner via the regulation of HIF-2α expression.
6. Hypoxia: A Novel Regulator of DUBs
As most of the important cellular enzymes, DUBs can be regulated on several different layers, including transcriptional regulation, mRNA stability and translation, protein stability, and catalytic activity. Despite increasing research about the function of different DUBs, knowledge about the regulation of DUBs in a cellular and physiological context is quite limited (Figure 8).
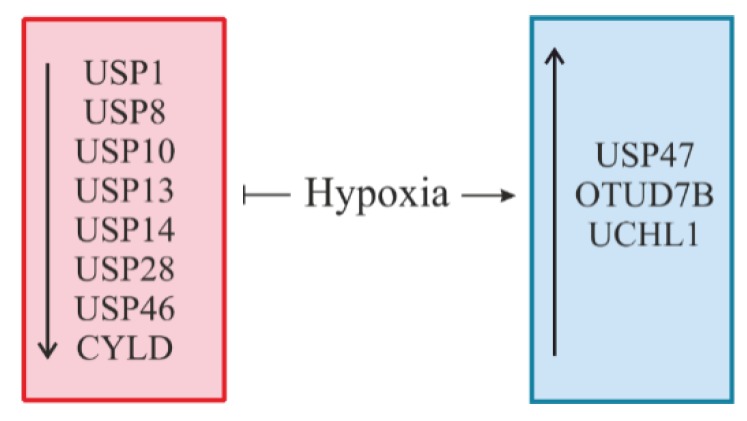
Figure 8.
Regulation of DUBs by hypoxia. Hypoxia can cause either a decrease in the level of certain DUBs (depicted in red) or an increase in the level of other DUBs (depicted in blue).
Taking into account that hypoxia is an indisputable cellular stressor, it is not unexpected that it is involved in the regulation of DUBs in order to adapt their functioning to the cell’s needs.
Although it cannot be precluded that hypoxia directly affects the activity or translation of DUBs, most regulation appears to take place at the mRNA level. It has been shown that exposure of human U87 glioma cells to hypoxia (3% O2) overnight diminishes mRNA levels of USP1, USP10, and USP14 [128]. USP1 is a negative regulator of DNA damage repair and deubiquitylates the monoubiquitylated Fanconi anemia group D2 protein (FANCD2) [129] and proliferating cell nuclear antigen (PCNA) [130]. USP10 is known to act as a tumor suppressor by stabilizing p53 and regulating MDM2-induced p53 nuclear export and degradation [131,132], and USP14 is required for the degradation of the chemokine receptor CXCR4 [133] and is able to inhibit endoplasmic reticulum-associated degradation (ERAD) via interaction with inositol-requiring enzyme-1α (IRE-1α) [134].
In addition, the expression of USP8, which has been shown to deubiquitylate HIF [72] and take part in EGFR turnover [73], was reported to be downregulated during intermittent hypoxia/reoxygenation conditions in renal tubular epithelial cells [135].
Further, decreased USP13 mRNA and protein expression could be detected after exposure of several melanoma cell lines to 2% O2 for 6 to 24 h. These changes appeared to be cell type and tissue-specific, since only melanoma cells (but not HEK293 or HeLa cells) displayed hypoxia-mediated USP13 reduction. Since USP13 limits the autodegradation of the E3 ubiquitin ligase SIAH2, decreased USP13 expression seen under hypoxia increased SIAH2 activity against its target substrates Sprouty2 and PHD3 [136]. In line with these results were findings where a depletion of USP13 in fibroblasts converted these fibroblasts into cells with a more aggressive phenotype with enhanced proliferative, migratory, and invasive capacities. Additionally, USP13 interacted with the tumor suppressor phosphatase and tensin homolog (PTEN). Downregulation of USP13 correlated with PTEN ubiquitylation and degradation in lung fibroblasts [137]. Hence, the hypoxia-induced decrease in USP13 expression may be pathogenically important during melanoma carcinogenesis and fibroblast conversion in idiopathic pulmonary fibrosis.
Hypoxia, as well as treatment with Ni compounds, contributed to the transcriptional repression of USP28 in A549 lung cancer cells via increased dimethylation of histone H3 lysine 9 at the USP28 promoter. Moreover, the authors showed that besides transcriptional regulation, a decrease in the USP28 protein level was also associated with increased protein degradation. In addition, a decrease in USP28 expression contributed to a higher level of c-MYC ubiquitylation and thus promoted degradation of this oncoprotein [138]. In contrast, the hypoxia-mediated USP28 decrease could not be observed in either MDA-MB-231 breast cancer cells or in mouse embryonic fibroblasts [117].
Further, an enhanced degradation of the PHLPP Ser/Thr protein phosphatase tumor suppressor appeared to be the result of reduced USP46 mRNA expression under hypoxic conditions in colon cancer cells. Although HIF-1α (but not HIF-2α) seemed to contribute to the reduction of PHLPP expression under hypoxia, it was mechanistically dependent on an mTOR-mediated decrease in protein translation. As a functional consequence of the hypoxia-reduced USP46 levels, the colon cancer cell lines SW480 and HCT116 showed increased chemotherapy resistance [139].
Interestingly, hypoxia-induced zinc finger protein SNAI1, together with hairy and enhancer of split-1 (HES1) (known as transcriptional repressors), might also be involved in the downregulation of another DUB, CYLD. This mechanism is likely to occur in hypoxia-mediated decreases in CYLD mRNA and protein levels in glioblastoma multiforme (GBM) [140]. CYLD overexpression was also shown to suppress the expression of various proinflammatory cytokines induced by TNF-α stimulation, suggesting that a hypoxia-mediated CYLD decrease could contribute to an increase in sensitivity to inflammatory stimuli in GBM cells. In line with this, it was also reported that hypoxia could promote an E6-dependent ubiquitylation and degradation of CYLD in HPV-infected cells, activating in turn the proinflammatory transcription factor NF-κB [141].
USP47, which is known to deubiquitylate β-catenin, thus increasing the proliferation of lung and prostate cancer cells [142], was reported to be upregulated under hypoxic conditions [143]. This upregulation, mediated by transcription factor SOX9, was found to promote the deubiquitylation of SNAI1 in three colorectal cancer cell lines, which boosted the endothelial-to-mesenchymal transition [143].
There is also evidence showing that hypoxia can induce OTUD7B via a p38 MAPK pathway [126] and UCHL1 expression involving a HIF-α-mediated mechanism [144]. It was reported that HIF-1α and HIF-2α promoted UCHL1 transcription by binding potential HRE sites within the UCHL1 promoter, therefore inducing cell apoptosis in hypoxia-induced neuronal injury following neuronal hypoxic ischemic encephalopathy [144].
Recent reports have also revealed that oxygen-regulated HIF hydroxylases could have an effect on the stability and/or activity of certain DUBs. For instance, oxygen-regulated FIH was reported to conduct hydroxylation of its substrate, the DUB OTUB1. Although FIH-promoted hydroxylation does not affect OTUB1 stability, it likely regulates cellular energy metabolism, which is reflected by altered phosphorylation of the AMP-activated protein kinase α (AMPKα). The molecular mechanisms underpinning this alteration in AMPKα phosphorylation remains so far unknown [145]. Moreover, it has been shown that PHD1 (EGLN2) can hydroxylate the transcription factor FOXO3a and thereby prevent it from interaction with USP9x [146], demonstrating an indirect effect of prolyl hydroxylation on a deubiquitinating enzyme.
7. Conclusions and Perspectives
Taking into account that the HIF pathway is a master regulator of cellular adaptation to hypoxia, the fact that it is tightly controlled in healthy cells in order to avoid its inappropriate activation does not come as a surprise. HIF signaling pathway dysfunction is usually observed in many tumors and is likely to promote and/or follow tumor growth and cancer development. HIF abundance in the cell is more likely to be regulated on its protein level via Ub-mediated degradation controlled by a great number of E3 ligases and DUBs (Table 1 and Table 2). One of the possible mechanisms leading to an unbalanced HIF-α level is the activity of specific DUBs and/or E3 ubiquitin ligases, some of which are reciprocally regulated by hypoxia. However, the current knowledge represents just a basic understanding of the interaction of DUBs and HIFs with different signaling pathways involved in a variety of diseases (cancer in particular). At the same time, the lack of knowledge increases the demand to further unravel the role of E3 ligases and DUBs and the mechanisms linking their action with HIF signaling to better understand the pathogenesis of cancer and its future therapies.
Acknowledgments
The authors are grateful to all researchers who contributed to the field and apologize to all those whose work could not be cited due to space limitations.
Author Contributions
K.K. and T.K. conceptualized and wrote the review.
Funding
This work was supported by grants from the Academy of Finland (SA 296027), the Jane and Aatos Erkko Foundation, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, Biocenter Oulu, and the European Cooperation in Science and Technology Organization (COST Action BM1203/EU-ROS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schubert U., Anton L.C., Gibbs J., Norbury C.C., Yewdell J.W., Bennink J.R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A. Intracellular protein degradation: From a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Best Pract. Res. Clin. Haematol. 2017;30:341–355. doi: 10.1016/j.beha.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Holkova B., Grant S. Proteasome inhibitors in mantle cell lymphoma. Best Pract. Res. Clin. Haematol. 2012;25:133–141. doi: 10.1016/j.beha.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau P., Richardson P.G., Cavo M., Orlowski R.Z., San Miguel J.F., Palumbo A., Harousseau J.L. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muz B., de la Puente P., Azab F., Azab A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soni S., Padwad Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017;56:503–515. doi: 10.1080/0284186X.2017.1301680. [DOI] [PubMed] [Google Scholar]
- 7.Haq S., Ramakrishna S. Deubiquitylation of deubiquitylases. Open Biol. 2017;7:170016. doi: 10.1098/rsob.170016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 9.Zheng N., Shabek N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017;86:129–157. doi: 10.1146/annurev-biochem-060815-014922. [DOI] [PubMed] [Google Scholar]
- 10.D’Arcy P., Wang X., Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol. Ther. 2015;147:32–54. doi: 10.1016/j.pharmthera.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 11.De Bie P., Ciechanover A. Ubiquitination of E3 ligases: Self-regulation of the ubiquitin system via proteolytic and non-proteolytic mechanisms. Cell Death Differ. 2011;18:1393–1402. doi: 10.1038/cdd.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akutsu M., Dikic I., Bremm A. Ubiquitin chain diversity at a glance. J. Cell Sci. 2016;129:875–880. doi: 10.1242/jcs.183954. [DOI] [PubMed] [Google Scholar]
- 13.Schnell J.D., Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay D., Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 15.Haglund K., Dikic I. Ubiquitylation and cell signaling. EMBO J. 2005;24:3353–3359. doi: 10.1038/sj.emboj.7600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P.P., Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 17.Huang F., Kirkpatrick D., Jiang X., Gygi S., Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Pickart C.M. Ubiquitin in chains. Trends Biochem. Sci. 2000;25:544–548. doi: 10.1016/S0968-0004(00)01681-9. [DOI] [PubMed] [Google Scholar]
- 19.Woelk T., Sigismund S., Penengo L., Polo S. The ubiquitination code: A signalling problem. Cell Div. 2007;2:11. doi: 10.1186/1747-1028-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J., Yan Q. Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Front. Oncol. 2012;2:26. doi: 10.3389/fonc.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach I., Ostendorff H.P. Orchestrating nuclear functions: Ubiquitin sets the rhythm. Trends Biochem. Sci. 2003;28:189–195. doi: 10.1016/S0968-0004(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 22.Huang T.T., D’Andrea A.D. Regulation of DNA repair by ubiquitylation. Nat. Rev. Mol. Cell Biol. 2006;7:323–334. doi: 10.1038/nrm1908. [DOI] [PubMed] [Google Scholar]
- 23.Huang H., Jeon M.S., Liao L., Yang C., Elly C., Yates J.R., 3rd, Liu Y.C. K33-linked polyubiquitination of T cell receptor-zeta regulates proteolysis-independent T cell signaling. Immunity. 2010;33:60–70. doi: 10.1016/j.immuni.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickliffe K.E., Williamson A., Meyer H.J., Kelly A., Rape M. K11-linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 2011;21:656–663. doi: 10.1016/j.tcb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durcan T.M., Tang M.Y., Perusse J.R., Dashti E.A., Aguileta M.A., McLelland G.L., Gros P., Shaler T.A., Faubert D., Coulombe B., et al. USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin. EMBO J. 2014;33:2473–2491. doi: 10.15252/embj.201489729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komander D., Clague M.J., Urbé S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 27.Fraile J.M., Quesada V., Rodriguez D., Freije J.M., Lopez-Otin C. Deubiquitinases in cancer: New functions and therapeutic options. Oncogene. 2012;31:2373–2388. doi: 10.1038/onc.2011.443. [DOI] [PubMed] [Google Scholar]
- 28.Abdul Rehman S.A., Kristariyanto Y.A., Choi S.Y., Nkosi P.J., Weidlich S., Labib K., Hofmann K., Kulathu Y. MINDY-1 Is a Member of an Evolutionarily Conserved and Structurally Distinct New Family of Deubiquitinating Enzymes. Mol. Cell. 2016;63:146–155. doi: 10.1016/j.molcel.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mevissen T.E.T., Komander D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017;86:159–192. doi: 10.1146/annurev-biochem-061516-044916. [DOI] [PubMed] [Google Scholar]
- 30.Heideker J., Wertz I.E. DUBs, the regulation of cell identity and disease. Biochem. J. 2015;465:1–26. doi: 10.1042/BJ20140496. [DOI] [PubMed] [Google Scholar]
- 31.Tian Z., D’Arcy P., Wang X., Ray A., Tai Y.T., Hu Y., Carrasco R.D., Richardson P., Linder S., Chauhan D., et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014;123:706–716. doi: 10.1182/blood-2013-05-500033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poondla N., Chandrasekaran A.P., Kim K.S., Ramakrishna S. Deubiquitinating enzymes as cancer biomarkers: New therapeutic opportunities? BMB Rep. 2019;52:181–189. doi: 10.5483/BMBRep.2019.52.3.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 35.Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 36.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992;12:5447–5454. doi: 10.1128/MCB.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian H., McKnight S.L., Russell D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 38.Gu Y.Z., Moran S.M., Hogenesch J.B., Wartman L., Bradfield C.A. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Exp. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 39.Bardos J.I., Ashcroft M. Hypoxia-inducible factor-1 and oncogenic signalling. Bioessays. 2004;26:262–269. doi: 10.1002/bies.20002. [DOI] [PubMed] [Google Scholar]
- 40.Choudhry H., Harris A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018;27:281–298. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Hirota K., Semenza G.L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol. Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Wigerup C., Pahlman S., Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Diebold I., Djordjevic T., Hess J., Görlach A. Rac-1 promotes pulmonary artery smooth muscle cell proliferation by upregulation of plasminogen activator inhibitor-1: Role of NFκB-dependent hypoxia-inducible factor-1α transcription. Thromb. Haemost. 2008;100:1021–1028. doi: 10.1160/TH08-07-0473. [DOI] [PubMed] [Google Scholar]
- 44.Düvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., Triantafellow E., Ma Q., Gorski R., Cleaver S., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang L.E., Gu J., Schau M., Bunn H.F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubbi M.E., Hu H., Kshitiz, Ahmed I., Levchenko A., Semenza G.L. Chaperone-mediated autophagy targets hypoxia-inducible factor-1alpha (HIF-1alpha) for lysosomal degradation. J. Biol. Chem. 2013;288:10703–10714. doi: 10.1074/jbc.M112.414771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masoud G.N., Li W. HIF-1alpha pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivan M., Kaelin W.G., Jr. The EGLN-HIF O2-Sensing System: Multiple Inputs and Feedbacks. Mol. Cell. 2017;66:772–779. doi: 10.1016/j.molcel.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson C.M., Ohh M. The multifaceted von Hippel-Lindau tumour suppressor protein. FEBS Lett. 2014;588:2704–2711. doi: 10.1016/j.febslet.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Lando D., Peet D.J., Whelan D.A., Gorman J.J., Whitelaw M.L. Asparagine hydroxylation of the HIF transactivation domain: A hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C., Peng Z., Zhu M., Wang P., Du X., Li X., Liu Y., Jin Y., McNutt M.A., Yin Y. USP9X destabilizes pVHL and promotes cell proliferation. Oncotarget. 2016;7:60519–60534. doi: 10.18632/oncotarget.11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen H.C., Yang H., Fribourgh J.L., Wolfe L.S., Xiong Y. Insights into Cullin-RING E3 ubiquitin ligase recruitment: Structure of the VHL-EloBC-Cul2 complex. Structure. 2015;23:441–449. doi: 10.1016/j.str.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kruizinga R.C., van Marion D.M., den Dunnen W.F., de Groot J.C., Hoving E.W., Oosting S.F., Timmer-Bosscha H., Derks R.P., Cornelissen C., van der Luijt R.B., et al. Difference in CXCR4 expression between sporadic and VHL-related hemangioblastoma. Fam. Cancer. 2016;15:607–616. doi: 10.1007/s10689-016-9879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aufforth R.D., Ramakant P., Sadowski S.M., Mehta A., Trebska-McGowan K., Nilubol N., Pacak K., Kebebew E. Pheochromocytoma Screening Initiation and Frequency in von Hippel-Lindau Syndrome. J. Clin. Endocrinol. Metab. 2015;100:4498–4504. doi: 10.1210/jc.2015-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanharanta S., Shu W., Brenet F., Hakimi A.A., Heguy A., Viale A., Reuter V.E., Hsieh J.J., Scandura J.M., Massague J. Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat. Med. 2013;19:50–56. doi: 10.1038/nm.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Na X., Wu G., Ryan C.K., Schoen S.R., di’Santagnese P.A., Messing E.M. Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J. Urol. 2003;170:588–592. doi: 10.1097/01.ju.0000074870.54671.98. [DOI] [PubMed] [Google Scholar]
- 57.Krieg M., Marti H.H., Plate K.H. Coexpression of erythropoietin and vascular endothelial growth factor in nervous system tumors associated with von Hippel-Lindau tumor suppressor gene loss of function. Blood. 1998;92:3388–3393. [PubMed] [Google Scholar]
- 58.Barth S., Nesper J., Hasgall P.A., Wirthner R., Nytko K.J., Edlich F., Katschinski D.M., Stiehl D.P., Wenger R.H., Camenisch G. The peptidyl prolyl cis/trans isomerase FKBP38 determines hypoxia-inducible transcription factor prolyl-4-hydroxylase PHD2 protein stability. Mol. Cell Biol. 2007;27:3758–3768. doi: 10.1128/MCB.01324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakayama K., Ronai Z. Siah: New players in the cellular response to hypoxia. Cell Cycle. 2004;3:1345–1347. doi: 10.4161/cc.3.11.1207. [DOI] [PubMed] [Google Scholar]
- 60.Fukuba H., Takahashi T., Jin H.G., Kohriyama T., Matsumoto M. Abundance of aspargynyl-hydroxylase FIH is regulated by Siah-1 under normoxic conditions. Neurosci. Lett. 2008;433:209–214. doi: 10.1016/j.neulet.2007.12.069. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L., Peng S., Dai X., Gan W., Nie X., Wei W., Hu G., Guo J. Tumor suppressor SPOP ubiquitinates and degrades EglN2 to compromise growth of prostate cancer cells. Cancer Lett. 2017;390:11–20. doi: 10.1016/j.canlet.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson J.E., III, Wu Y., Smith K., Charles P., Powers K., Wang H., Patterson C. ASB4 is a hydroxylation substrate of FIH and promotes vascular differentiation via an oxygen-dependent mechanism. Mol. Cell Biol. 2007;27:6407–6419. doi: 10.1128/MCB.00511-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z., Wang D., Messing E.M., Wu G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 2005;6:373–378. doi: 10.1038/sj.embor.7400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azenha D., Lopes M.C., Martins T.C. Claspin functions in cell homeostasis-A link to cancer? DNA Repair. 2017;59:27–33. doi: 10.1016/j.dnarep.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Wang C., Yang C., Ji J., Jiang J., Shi M., Cai Q., Yu Y., Zhu Z., Zhang J. Deubiquitinating enzyme USP20 is a positive regulator of Claspin and suppresses the malignant characteristics of gastric cancer cells. Int. J. Oncol. 2017;50:1136–1146. doi: 10.3892/ijo.2017.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan J., Luo K., Deng M., Li Y., Yin P., Gao B., Fang Y., Wu P., Liu T., Lou Z. HERC2-USP20 axis regulates DNA damage checkpoint through Claspin. Nucleic Acids Res. 2014;42:13110–13121. doi: 10.1093/nar/gku1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu C., Luo K., Zhao F., Yin P., Song Y., Deng M., Huang J., Chen Y., Li L., Lee S., et al. USP20 positively regulates tumorigenesis and chemoresistance through beta-catenin stabilization. Cell Death Differ. 2018;25:1855–1869. doi: 10.1038/s41418-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roy A., Zhang M., Saad Y., Kolattukudy P.E. Antidicer RNAse activity of monocyte chemotactic protein-induced protein-1 is critical for inducing angiogenesis. Am. J. Physiol. Cell Physiol. 2013;305:C1021–C1032. doi: 10.1152/ajpcell.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun P., Lu Y.X., Cheng D., Zhang K., Zheng J., Liu Y., Wang X., Yuan Y.F., Tang Y.D. Monocyte Chemoattractant Protein-Induced Protein 1 Targets Hypoxia-Inducible Factor 1alpha to Protect Against Hepatic Ischemia/Reperfusion Injury. Hepatology. 2018;68:2359–2375. doi: 10.1002/hep.30086. [DOI] [PubMed] [Google Scholar]
- 70.Ligeza J., Marona P., Gach N., Lipert B., Miekus K., Wilk W., Jaszczynski J., Stelmach A., Loboda A., Dulak J., et al. MCPIP1 contributes to clear cell renal cell carcinomas development. Angiogenesis. 2017;20:325–340. doi: 10.1007/s10456-017-9540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu W., Ning H., Gu L., Peng H., Wang Q., Hou R., Fu M., Hoft D.F., Liu J. MCPIP1 Selectively Destabilizes Transcripts Associated with an Antiapoptotic Gene Expression Program in Breast Cancer Cells That Can Elicit Complete Tumor Regression. Cancer Res. 2016;76:1429–1440. doi: 10.1158/0008-5472.CAN-15-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troilo A., Alexander I., Muehl S., Jaramillo D., Knobeloch K., Krek W. HIF1α deubiquitination by USP8 is essential for ciliogenesis in normoxia. EMBO Rep. 2014;15:77–85. doi: 10.1002/embr.201337688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alwan H.A., van Leeuwen J.E. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J. Biol. Chem. 2007;282:1658–1669. doi: 10.1074/jbc.M604711200. [DOI] [PubMed] [Google Scholar]
- 74.Reincke M., Sbiera S., Hayakawa A., Theodoropoulou M., Osswald A., Beuschlein F., Meitinger T., Mizuno-Yamasaki E., Kawaguchi K., Saeki Y., et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 2015;47:31–38. doi: 10.1038/ng.3166. [DOI] [PubMed] [Google Scholar]
- 75.Dupont S., Mamidi A., Cordenonsi M., Montagner M., Zacchigna L., Adorno M., Martello G., Stinchfield M.J., Soligo S., Morsut L., et al. FAM/USP9x, a Deubiquitinating Enzyme Essential for TGFß Signaling, Controls Smad4 Monoubiquitination. Cell. 2009;136:123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 76.Nagai H., Noguchi T., Homma K., Katagiri K., Takeda K., Matsuzawa A., Ichijo H. Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death. Mol. Cell. 2009;36:805–818. doi: 10.1016/j.molcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Perez-Mancera P.A., Rust A.G., van der Weyden L., Kristiansen G., Li A., Sarver A.L., Silverstein K.A., Grutzmann R., Aust D., Rummele P., et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwickart M., Huang X., Lill J.R., Liu J., Ferrando R., French D.M., Maecker H., O’Rourke K., Bazan F., Eastham-Anderson J., et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 79.Goto Y., Zeng L., Yeom C.J., Zhu Y., Morinibu A., Shinomiya K., Kobayashi M., Hirota K., Itasaka S., Yoshimura M., et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1alpha. Nat. Commun. 2015;6:6153. doi: 10.1038/ncomms7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castegna A., Aksenov M., Aksenova M., Thongboonkerd V., Klein J.B., Pierce W.M., Booze R., Markesbery W.R., Butterfield D.A. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic. Biol. Med. 2002;33:562–571. doi: 10.1016/S0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 81.Di Domenico F., Coccia R., Cocciolo A., Murphy M.P., Cenini G., Head E., Butterfield D.A., Giorgi A., Schinina M.E., Mancuso C., et al. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer’s disease neuropathology: Redox proteomics analysis of human brain. Biochim. Biophys. Acta. 2013;1832:1249–1259. doi: 10.1016/j.bbadis.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu Y.Y., Yang M., Zhao M., Luo Q., Yang L., Peng H., Wang J., Huang S.K., Zheng Z.X., Yuan X.H., et al. The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2 pathways. Tumour Biol. 2015;36:8379–8387. doi: 10.1007/s13277-015-3566-0. [DOI] [PubMed] [Google Scholar]
- 83.Hussain S., Bedekovics T., Chesi M., Bergsagel P.L., Galardy P.J. UCHL1 is a biomarker of aggressive multiple myeloma required for disease progression. Oncotarget. 2015;6:40704–40718. doi: 10.18632/oncotarget.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdelmaksoud-Dammak R., Saadallah-Kallel A., Miladi-Abdennadher I., Ayedi L., Khabir A., Sallemi-Boudawara T., Frikha M., Daoud J., Mokdad-Gargouri R. CpG methylation of ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) and P53 mutation pattern in sporadic colorectal cancer. Tumour Biol. 2016;37:1707–1714. doi: 10.1007/s13277-015-3902-4. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima R., Goto Y., Koyasu S., Kobayashi M., Morinibu A., Yoshimura M., Hiraoka M., Hammond E.M., Harada H. UCHL1-HIF-1 axis-mediated antioxidant property of cancer cells as a therapeutic target for radiosensitization. Sci. Rep. 2017;7:6879. doi: 10.1038/s41598-017-06605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Fallon L., Lashuel H.A., Liu Z., Lansbury P.T., Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/S0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y.V., Baek J.H., Zhang H., Diez R., Cole R.N., Semenza G.L. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ehrlich E.S., Wang T., Luo K., Xiao Z., Niewiadomska A.M., Martinez T., Xu W., Neckers L., Yu X.F. Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA. 2009;106:20330–20335. doi: 10.1073/pnas.0810571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y.V., Hubbi M.E., Pan F., McDonald K.R., Mansharamani M., Cole R.N., Liu J.O., Semenza G.L. Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J. Biol. Chem. 2007;282:37064–37073. doi: 10.1074/jbc.M705015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amir S., Wang R., Simons J.W., Mabjeesh N.J. SEPT9_v1 up-regulates hypoxia-inducible factor 1 by preventing its RACK1-mediated degradation. J. Biol. Chem. 2009;284:11142–11151. doi: 10.1074/jbc.M808348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paatero I., Jokilammi A., Heikkinen P.T., Iljin K., Kallioniemi O., Jones F.E., Jaakkola P.M., Elenius K. Interaction with ErbB4 promotes hypoxia-inducible factor-1α signaling. J. Biol. Chem. 2012;287:9659–9671. doi: 10.1074/jbc.M111.299537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baek J.H., Liu Y.V., McDonald K.R., Wesley J.B., Zhang H., Semenza G.L. Spermidine/spermine N(1)-acetyltransferase-1 binds to hypoxia-inducible factor-1alpha (HIF-1alpha) and RACK1 and promotes ubiquitination and degradation of HIF-1alpha. J. Biol. Chem. 2007;282:33358–33366. doi: 10.1074/jbc.M705627200. [DOI] [PubMed] [Google Scholar]
- 93.Baek J.H., Liu Y.V., McDonald K.R., Wesley J.B., Hubbi M.E., Byun H., Semenza G.L. Spermidine/spermine-N1-acetyltransferase 2 is an essential component of the ubiquitin ligase complex that regulates hypoxia-inducible factor 1alpha. J. Biol. Chem. 2007;282:23572–23580. doi: 10.1074/jbc.M703504200. [DOI] [PubMed] [Google Scholar]
- 94.Bento C.F., Fernandes R., Ramalho J., Marques C., Shang F., Taylor A., Pereira P. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1alpha for degradation in the presence of methylglyoxal. PLoS ONE. 2010;5:e15062. doi: 10.1371/journal.pone.0015062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo W., Zhong J., Chang R., Hu H., Pandey A., Semenza G.L. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha. J. Biol. Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferreira J.V., Soares A.R., Ramalho J.S., Pereira P., Girao H. K63 linked ubiquitin chain formation is a signal for HIF1A degradation by Chaperone-Mediated Autophagy. Sci. Rep. 2015;5:10210. doi: 10.1038/srep10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferreira J.V., Fofo H., Bejarano E., Bento C.F., Ramalho J.S., Girao H., Pereira P. STUB1/CHIP is required for HIF1A degradation by chaperone-mediated autophagy. Autophagy. 2013;9:1349–1366. doi: 10.4161/auto.25190. [DOI] [PubMed] [Google Scholar]
- 98.Joshi S., Singh A.R., Durden D.L. MDM2 regulates hypoxic hypoxia-inducible factor 1alpha stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. J. Biol. Chem. 2014;289:22785–22797. doi: 10.1074/jbc.M114.587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ravi R., Mookerjee B., Bhujwalla Z.M., Sutter C.H., Artemov D., Zeng Q., Dillehay L.E., Madan A., Semenza G.L., Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 100.Amelio I., Inoue S., Markert E.K., Levine A.J., Knight R.A., Mak T.W., Melino G. TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1alpha degradation. Proc. Natl. Acad. Sci. USA. 2015;112:226–231. doi: 10.1073/pnas.1410609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koh M.Y., Lemos R., Jr., Liu X., Powis G. The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koh M.Y., Darnay B.G., Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol. Cell Biol. 2008;28:7081–7095. doi: 10.1128/MCB.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koh M.Y., Nguyen V., Lemos R., Jr., Darnay B.G., Kiriakova G., Abdelmelek M., Ho T.H., Karam J., Monzon F.A., Jonasch E., et al. Hypoxia-induced SUMOylation of E3 ligase HAF determines specific activation of HIF2 in clear-cell renal cell carcinoma. Cancer Res. 2015;75:316–329. doi: 10.1158/0008-5472.CAN-13-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun H., Li X.B., Meng Y., Fan L., Li M., Fang J. TRAF6 upregulates expression of HIF-1alpha and promotes tumor angiogenesis. Cancer Res. 2013;73:4950–4959. doi: 10.1158/0008-5472.CAN-13-0370. [DOI] [PubMed] [Google Scholar]
- 105.Rezaeian A.H., Li C.F., Wu C.Y., Zhang X., Delacerda J., You M.J., Han F., Cai Z., Jeong Y.S., Jin G., et al. A hypoxia-responsive TRAF6-ATM-H2AX signalling axis promotes HIF1alpha activation, tumorigenesis and metastasis. Nat. Cell Biol. 2017;19:38–51. doi: 10.1038/ncb3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang S., Wang B., Humphries F., Hogan A.E., O’Shea D., Moynagh P.N. The E3 ubiquitin ligase Pellino3 protects against obesity-induced inflammation and insulin resistance. Immunity. 2014;41:973–987. doi: 10.1016/j.immuni.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Yuan W.C., Lee Y.R., Huang S.F., Lin Y.M., Chen T.Y., Chung H.C., Tsai C.H., Chen H.Y., Chiang C.T., Lai C.K., et al. A Cullin3-KLHL20 Ubiquitin ligase-dependent pathway targets PML to potentiate HIF-1 signaling and prostate cancer progression. Cancer Cell. 2011;20:214–228. doi: 10.1016/j.ccr.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 108.Woik N., Dietz C.T., Schaker K., Kroll J. Kelch-like ECT2-interacting protein KLEIP regulates late-stage pulmonary maturation via Hif-2alpha in mice. Dis. Model. Mech. 2014;7:683–692. doi: 10.1242/dmm.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang H.J., Kim H.J., Rih J.K., Mattson T.L., Kim K.W., Cho C.H., Isaacs J.S., Bae I. BRCA1 plays a role in the hypoxic response by regulating HIF-1alpha stability and by modulating vascular endothelial growth factor expression. J. Biol. Chem. 2006;281:13047–13056. doi: 10.1074/jbc.M513033200. [DOI] [PubMed] [Google Scholar]
- 110.Flugel D., Gorlach A., Michiels C., Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol. Cell Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flugel D., Gorlach A., Kietzmann T. GSK-3beta regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1alpha. Blood. 2012;119:1292–1301. doi: 10.1182/blood-2011-08-375014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cassavaugh J.M., Hale S.A., Wellman T.L., Howe A.K., Wong C., Lounsbury K.M. Negative regulation of HIF-1alpha by an FBW7-mediated degradation pathway during hypoxia. J. Cell Biochem. 2011;112:3882–3890. doi: 10.1002/jcb.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Popov N., Wanzel M., Madiredjo M., Zhang D., Beijersbergen R., Bernards R., Moll R., Elledge S.J., Eilers M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 2007;9:765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]
- 114.Diefenbacher M.E., Popov N., Blake S.M., Schülein-Völk C., Nye E., Spencer-Dene B., Jaenicke L.A., Eilers M., Behrens A. The deubiquitinase USP28 controls intestinal homeostasis and promotes Colorectal cancer. J. Clin. Investig. 2014;124:3407–3418. doi: 10.1172/JCI73733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo G., Xu Y., Gong M., Cao Y., An R. USP28 is a potential prognostic marker for bladder cancer. Tumour Biol. 2014;35:4017–4022. doi: 10.1007/s13277-013-1525-1. [DOI] [PubMed] [Google Scholar]
- 116.Zhao L.J., Zhang T., Feng X.J., Chang J., Suo F.Z., Ma J.L., Liu Y.J., Liu Y., Zheng Y.C., Liu H.M. USP28 contributes to the proliferation and metastasis of gastric cancer. J. Cell Biochem. 2018 doi: 10.1002/jcb.28040. [DOI] [PubMed] [Google Scholar]
- 117.Richter K., Paakkola T., Mennerich D., Kubaichuk K., Konzack A., Kippari H.A., Kozlova N., Koivunen P., Haapasaari K.M., Jukkola-Vuorinen A., et al. USP28 Deficiency Promotes Breast and Liver Carcinogenesis as well as Tumor Angiogenesis in a HIF-independent Manner. Mol. Cancer Res. 2018;16:1000–1012. doi: 10.1158/1541-7786.MCR-17-0452. [DOI] [PubMed] [Google Scholar]
- 118.Li M., Brooks C.L., Kon N., Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell. 2004;13:879–886. doi: 10.1016/S1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 119.Wu H.T., Kuo Y.C., Hung J.J., Huang C.H., Chen W.Y., Chou T.Y., Chen Y., Chen Y.J., Chen Y.J., Cheng W.C., et al. K63-polyubiquitinated HAUSP deubiquitinates HIF-1alpha and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat. Commun. 2016;7:13644. doi: 10.1038/ncomms13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hassink G.C., Zhao B., Sompallae R., Altun M., Gastaldello S., Zinin N.V., Masucci M.G., Lindsten K. The ER-resident ubiquitin-specific protease 19 participates in the UPR and rescues ERAD substrates. EMBO Rep. 2009;10:755–761. doi: 10.1038/embor.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakayama K., Frew I.J., Hagensen M., Skals M., Habelhah H., Bhoumik A., Kadoya T., Erdjument-Bromage H., Tempst P., Frappell P.B., et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Q., Wang Z., Hou F., Harding R., Huang X., Dong A., Walker J.R., Tong Y. The substrate binding domains of human SIAH E3 ubiquitin ligases are now crystal clear. Biochim. Biophys. Acta Gen. Subj. 2017;1861:3095–3105. doi: 10.1016/j.bbagen.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 123.Altun M., Zhao B., Velasco K., Liu H., Hassink G., Paschke J., Pereira T., Lindsten K. Ubiquitin-specific protease 19 (USP19) regulates hypoxia-inducible factor 1alpha (HIF-1alpha) during hypoxia. J. Biol. Chem. 2012;287:1962–1969. doi: 10.1074/jbc.M111.305615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bett J.S., Ibrahim A.F., Garg A.K., Kelly V., Pedrioli P., Rocha S., Hay R.T. The P-body component USP52/PAN2 is a novel regulator of HIF1A mRNA stability. Biochem. J. 2013;451:185–194. doi: 10.1042/BJ20130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bremm A., Moniz S., Mader J., Rocha S., Komander D. Cezanne (OTUD7B) regulates HIF-1α homeostasis in a proteasome-independent manner. EMBO Rep. 2014;15:1268–1277. doi: 10.15252/embr.201438850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luong le A., Fragiadaki M., Smith J., Boyle J., Lutz J., Dean J.L., Harten S., Ashcroft M., Walmsley S.R., Haskard D.O., et al. Cezanne regulates inflammatory responses to hypoxia in endothelial cells by targeting TRAF6 for deubiquitination. Circ. Res. 2013;112:1583–1591. doi: 10.1161/CIRCRESAHA.111.300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moniz S., Bandarra D., Biddlestone J., Campbell K.J., Komander D., Bremm A., Rocha S. Cezanne regulates E2F1-dependent HIF2alpha expression. J. Cell Sci. 2015;128:3082–3093. doi: 10.1242/jcs.168864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Minchenko O.H., Tsymbal D.O., Minchenko D.O., Riabovol O.O., Halkin O.V., Ratushna O.O. IRE-1α regulates expression of ubiquitin specific peptidases during hypoxic response in U87 glioma cells. Endoplasm. Reticul. Stress Dis. 2016;3:50–62. [Google Scholar]
- 129.Nijman S.M.B., Huang T.T., Dirac A.M.G., Brummelkamp T.R., Kerkhoven R.M., D’Andrea A.D., Bernards R. The deubiquitinating enzyme USP1 regulates the fanconi anemia pathway. Mol. Cell. 2005;17:331–339. doi: 10.1016/j.molcel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 130.Huang T.T., Nijman S.M.B., Mirchandani K.D., Galardy P.J., Cohn M.A., Haas W., Gygi S.P., Ploegh H.L., Bernards R., D’Andrea A.D. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 2006;8:339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 131.Lin Z., Yang H., Tan C., Li J., Liu Z., Quan Q., Kong S., Ye J., Gao B., Fang D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5:1639–1649. doi: 10.1016/j.celrep.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan J., Luo K., Zhang L., Cheville J.C., Lou Z. USP10 Regulates p53 Localization and Stability by Deubiquitinating p53. Cell. 2010;140:384–396. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mines M.A., Goodwin J.S., Limbird L.E., Cui F.F., Fan G.H. Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation. J. Biol. Chem. 2009;284:5742–5752. doi: 10.1074/jbc.M808507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nagai A., Kadowaki H., Maruyama T., Takeda K., Nishitoh H., Ichijo H. USP14 inhibits ER-associated degradation via interaction with IRE1alpha. Biochem. Biophys. Res. Commun. 2009;379:995–1000. doi: 10.1016/j.bbrc.2008.12.182. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y., Luo Y., Wang Y., Liu H., Yang Y., Wang Q. Effect of deubiquitinase USP8 on hypoxia/reoxygenationinduced inflammation by deubiquitination of TAK1 in renal tubular epithelial cells. Int. J. Mol. Med. 2018;42:3467–3476. doi: 10.3892/ijmm.2018.3881. [DOI] [PubMed] [Google Scholar]
- 136.Scortegagna M., Subtil T., Qi J., Kim H., Zhao W., Gu W., Kluger H., Ronai Z.A. USP13 enzyme regulates Siah2 ligase stability and activity via noncatalytic ubiquitin-binding domains. J. Biol. Chem. 2011;286:27333–27341. doi: 10.1074/jbc.M111.218214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Geng J., Huang X., Li Y., Xu X., Li S., Jiang D., Liang J., Jiang D., Wang C., Dai H. Down-regulation of USP13 mediates phenotype transformation of fibroblasts in idiopathic pulmonary fibrosis. Respir. Res. 2015;16:124. doi: 10.1186/s12931-015-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Q., Kluz T., Sun H., Costa M. Mechanisms of c-myc degradation by nickel compounds and hypoxia. PLoS ONE. 2009;4:e8531. doi: 10.1371/journal.pone.0008531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wen Y.A., Stevens P.D., Gasser M.L., Andrei R., Gao T. Downregulation of PHLPP expression contributes to hypoxia-induced resistance to chemotherapy in colon cancer cells. Mol. Cell Biol. 2013;33:4594–4605. doi: 10.1128/MCB.00695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo J., Shinriki S., Su Y., Nakamura T., Hayashi M., Tsuda Y., Murakami Y., Tasaki M., Hide T., Takezaki T., et al. Hypoxia suppresses cylindromatosis (CYLD) expression to promote inflammation in glioblastoma: Possible link to acquired resistance to anti-VEGF therapy. Oncotarget. 2014;5:6353–6364. doi: 10.18632/oncotarget.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.An J., Mo D., Liu H., Veena M.S., Srivatsan E.S., Massoumi R., Rettig M.B. Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-kappaB activation. Cancer Cell. 2008;14:394–407. doi: 10.1016/j.ccr.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi J., Liu Y., Xu X., Zhang W., Yu T., Jia J., Liu C. Deubiquitinase USP47/UBP64E Regulates beta-Catenin Ubiquitination and Degradation and Plays a Positive Role in Wnt Signaling. Mol. Cell Biol. 2015;35:3301–3311. doi: 10.1128/MCB.00373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi B.J., Park S.A., Lee S.Y., Cha Y.N., Surh Y.J. Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: A potential role of Sox9. Sci. Rep. 2017;7:15918. doi: 10.1038/s41598-017-15139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu H., Ying W., Wang W., Li W., Feng X. HIF1α and HIF2α mediated UCHL1 upregulation in hypoxia-induced neuronal injury following neuronal hypoxic ischemic encephalopathy. Int. J. Clin. Exp. Pathol. 2016;9:2677–2685. [Google Scholar]
- 145.Scholz C.C., Rodriguez J., Pickel C., Burr S., Fabrizio J.A., Nolan K.A., Spielmann P., Cavadas M.A., Crifo B., Halligan D.N., et al. FIH Regulates Cellular Metabolism through Hydroxylation of the Deubiquitinase OTUB1. PLoS Biol. 2016;14:e1002347. doi: 10.1371/journal.pbio.1002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng X., Zhai B., Koivunen P., Shin S.J., Lu G., Liu J., Geisen C., Chakraborty A.A., Moslehi J.J., Smalley D.M., et al. Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Genes Dev. 2014;28:1429–1444. doi: 10.1101/gad.242131.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ohh M., Park C.W., Ivan M., Hoffman M.A., Kim T.Y., Huang L.E., Pavletich N., Chau V., Kaelin W.G. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 148.Confalonieri S., Quarto M., Goisis G., Nuciforo P., Donzelli M., Jodice G., Pelosi G., Viale G., Pece S., Di Fiore P.P. Alterations of ubiquitin ligases in human cancer and their association with the natural history of the tumor. Oncogene. 2009;28:2959–2968. doi: 10.1038/onc.2009.156. [DOI] [PubMed] [Google Scholar]
- 149.Liu X.K., Li Q., Xu L.H., Hu L.J., Liao W.G., Zhang X.R., Liu Z.M., Wu D., Zeng M.S. Expression and clinical significance of SIAH in laryngeal squamous cell carcinoma. Med. Oncol. 2013;30:485. doi: 10.1007/s12032-013-0485-z. [DOI] [PubMed] [Google Scholar]
- 150.Kim C.J., Cho Y.G., Park C.H., Jeong S.W., Nam S.W., Kim S.Y., Lee S.H., Yoo N.J., Lee J.Y., Park W.S. Inactivating mutations of the Siah-1 gene in gastric cancer. Oncogene. 2004;23:8591–8596. doi: 10.1038/sj.onc.1208113. [DOI] [PubMed] [Google Scholar]
- 151.Yoshibayashi H., Okabe H., Satoh S., Hida K., Kawashima K., Hamasu S., Nomura A., Hasegawa S., Ikai I., Sakai Y. SIAH1 causes growth arrest and apoptosis in hepatoma cells through beta-catenin degradation-dependent and -independent mechanisms. Oncol. Rep. 2007;17:549–556. [PubMed] [Google Scholar]
- 152.Chan P., Moller A., Liu M.C., Sceneay J.E., Wong C.S., Waddell N., Huang K.T., Dobrovic A., Millar E.K., O’Toole S.A., et al. The expression of the ubiquitin ligase SIAH2 (seven in absentia homolog 2) is mediated through gene copy number in breast cancer and is associated with a basal-like phenotype and p53 expression. Breast Cancer Res. 2011;13:R19. doi: 10.1186/bcr2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brauckhoff A., Malz M., Tschaharganeh D., Malek N., Weber A., Riener M.O., Soll C., Samarin J., Bissinger M., Schmidt J., et al. Nuclear expression of the ubiquitin ligase seven in absentia homolog (SIAH)-1 induces proliferation and migration of liver cancer cells. J. Hepatol. 2011;55:1049–1057. doi: 10.1016/j.jhep.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 154.Li G., Ci W., Karmakar S., Chen K., Dhar R., Fan Z., Guo Z., Zhang J., Ke Y., Wang L., et al. SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer. Cancer Cell. 2014;25:455–468. doi: 10.1016/j.ccr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo J., Bao Y.C., Ji X.X., Chen B., Deng Q.F., Zhou S.W. SPOP promotes SIRT2 degradation and suppresses non-small cell lung cancer cell growth. Biochem. Biophys. Res. Commun. 2017;483:880–884. doi: 10.1016/j.bbrc.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 156.Geng C., He B., Xu L., Barbieri C.E., Eedunuri V.K., Chew S.A., Zimmermann M., Bond R., Shou J., Li C., et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc. Natl. Acad. Sci. USA. 2013;110:6997–7002. doi: 10.1073/pnas.1304502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Byun S., Lee S.Y., Lee J., Jeong C.H., Farrand L., Lim S., Reddy K., Kim J.Y., Lee M.H., Lee H.J., et al. USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin. Cancer Res. 2013;19:3894–3904. doi: 10.1158/1078-0432.CCR-12-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yan M., Zhao C., Wei N., Wu X., Cui J., Xing Y. High Expression of Ubiquitin-Specific Protease 8 (USP8) Is Associated with Poor Prognosis in Patients with Cervical Squamous Cell Carcinoma. Med. Sci. Monit. 2018;24:4934–4943. doi: 10.12659/MSM.909235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu L., Yao D., Zhang P., Ding W., Zhang X., Zhang C., Gong S., Zhang Y., Wang J., Sun T., et al. Deubiquitinase USP9X promotes cell migration, invasion and inhibits apoptosis of human pancreatic cancer. Oncol. Rep. 2017;38:3531–3537. doi: 10.3892/or.2017.6050. [DOI] [PubMed] [Google Scholar]
- 160.Fu X., Xie W., Song X., Wu K., Xiao L., Liu Y., Zhang L. Aberrant expression of deubiquitylating enzyme USP9X predicts poor prognosis in gastric cancer. Clin. Res. Hepatol. Gastroenterol. 2017;41:687–692. doi: 10.1016/j.clinre.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 161.Li X., Song N., Liu L., Liu X., Ding X., Song X., Yang S., Shan L., Zhou X., Su D., et al. USP9X regulates centrosome duplication and promotes breast carcinogenesis. Nat. Commun. 2017;8:14866. doi: 10.1038/ncomms14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Khan O.M., Carvalho J., Spencer-Dene B., Mitter R., Frith D., Snijders A.P., Wood S.A., Behrens A. The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J. Clin. Investig. 2018;128:1326–1337. doi: 10.1172/JCI97325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marona P., Gorka J., Mazurek Z., Wilk W., Rys J., Majka M., Jura J., Miekus K. MCPIP1 Downregulation in Clear Cell Renal Cell Carcinoma Promotes Vascularization and Metastatic Progression. Cancer Res. 2017;77:4905–4920. doi: 10.1158/0008-5472.CAN-16-3190. [DOI] [PubMed] [Google Scholar]
- 164.Chen G., Gharib T.G., Huang C.C., Thomas D.G., Shedden K.A., Taylor J.M., Kardia S.L., Misek D.E., Giordano T.J., Iannettoni M.D., et al. Proteomic analysis of lung adenocarcinoma: Identification of a highly expressed set of proteins in tumors. Clin. Cancer Res. 2002;8:2298–2305. [PubMed] [Google Scholar]
- 165.Jin C., Yu W., Lou X., Zhou F., Han X., Zhao N., Lin B. UCHL1 Is a Putative Tumor Suppressor in Ovarian Cancer Cells and Contributes to Cisplatin Resistance. J. Cancer. 2013;4:662–670. doi: 10.7150/jca.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu X., Zhu M., Yang X., Wang Y., Qin B., Cui C., Chen H., Sang A. Inhibition of RACK1 ameliorates choroidal neovascularization formation in vitro and in vivo. Exp. Mol. Pathol. 2016;100:451–459. doi: 10.1016/j.yexmp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 167.Shi S., Deng Y.Z., Zhao J.S., Ji X.D., Shi J., Feng Y.X., Li G., Li J.J., Zhu D., Koeffler H.P., et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J. Biol. Chem. 2012;287:7845–7858. doi: 10.1074/jbc.M111.315416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fei L., Ma Y., Zhang M., Liu X., Luo Y., Wang C., Zhang H., Zhang W., Han Y. RACK1 promotes lung cancer cell growth via an MCM7/RACK1/ Akt signaling complex. Oncotarget. 2017;8:40501–40513. doi: 10.18632/oncotarget.17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhong X., Li M., Nie B., Wu F., Zhang L., Wang E., Han Y. Overexpressions of RACK1 and CD147 associated with poor prognosis in stage T1 pulmonary adenocarcinoma. Ann. Surg. Oncol. 2013;20:1044–1052. doi: 10.1245/s10434-012-2377-4. [DOI] [PubMed] [Google Scholar]
- 170.Guo Y., Wang W., Wang J., Feng J., Wang Q., Jin J., Lv M., Li X., Li Y., Ma Y., et al. Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology. 2013;57:140–151. doi: 10.1002/hep.25978. [DOI] [PubMed] [Google Scholar]
- 171.Cao X.X., Xu J.D., Liu X.L., Xu J.W., Wang W.J., Li Q.Q., Chen Q., Xu Z.D., Liu X.P. RACK1: A superior independent predictor for poor clinical outcome in breast cancer. Int. J. Cancer. 2010;127:1172–1179. doi: 10.1002/ijc.25120. [DOI] [PubMed] [Google Scholar]
- 172.Peng R., Jiang B., Ma J., Ma Z., Wan X., Liu H., Chen Z., Cheng Q., Chen R. Forced downregulation of RACK1 inhibits glioma development by suppressing Src/Akt signaling activity. Oncol. Rep. 2013;30:2195–2202. doi: 10.3892/or.2013.2723. [DOI] [PubMed] [Google Scholar]
- 173.Deng Y.Z., Yao F., Li J.J., Mao Z.F., Hu P.T., Long L.Y., Li G., Ji X.D., Shi S., Guan D.X., et al. RACK1 suppresses gastric tumorigenesis by stabilizing the beta-catenin destruction complex. Gastroenterology. 2012;142:812–823. doi: 10.1053/j.gastro.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 174.Liang Z.L., Kim M., Huang S.M., Lee H.J., Kim J.M. Expression of carboxyl terminus of Hsp70-interacting protein (CHIP) indicates poor prognosis in human gallbladder carcinoma. Oncol. Lett. 2013;5:813–818. doi: 10.3892/ol.2013.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wen J., Luo K.J., Hu Y., Yang H., Fu J.H. Metastatic lymph node CHIP expression is a potential prognostic marker for resected esophageal squamous cell carcinoma patients. Ann. Surg. Oncol. 2013;20:1668–1675. doi: 10.1245/s10434-012-2733-4. [DOI] [PubMed] [Google Scholar]
- 176.Cheng L., Zang J., Dai H.J., Li F., Guo F. Ubiquitin ligase CHIP functions as an oncogene and activates the AKT signaling pathway in prostate cancer. Int. J. Oncol. 2018;53:203–214. doi: 10.3892/ijo.2018.4377. [DOI] [PubMed] [Google Scholar]
- 177.Patani N., Jiang W., Newbold R., Mokbel K. Prognostic implications of carboxyl-terminus of Hsc70 interacting protein and lysyl-oxidase expression in human breast cancer. J. Carcinog. 2010;9:9. doi: 10.4103/1477-3163.72505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang S., Wu X., Zhang J., Chen Y., Xu J., Xia X., He S., Qiang F., Li A., Shu Y., et al. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human gastric cancer. Gut. 2013;62:496–508. doi: 10.1136/gutjnl-2011-301522. [DOI] [PubMed] [Google Scholar]
- 179.Wang T., Yang J., Xu J., Li J., Cao Z., Zhou L., You L., Shu H., Lu Z., Li H., et al. CHIP is a novel tumor suppressor in pancreatic cancer through targeting EGFR. Oncotarget. 2014;5:1969–1986. doi: 10.18632/oncotarget.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang Y., Ren F., Wang Y., Feng Y., Wang D., Jia B., Qiu Y., Wang S., Yu J., Sung J.J., et al. CHIP/Stub1 functions as a tumor suppressor and represses NF-kappaB-mediated signaling in colorectal cancer. Carcinogenesis. 2014;35:983–991. doi: 10.1093/carcin/bgt393. [DOI] [PubMed] [Google Scholar]
- 181.Qian T., Wang J., Wang Q., Liu Y., Yu S., Wang Z., Sun D., Wang S. CHIP involves in non-small cell lung cancer prognosis through VEGF pathway. Biomed. Pharmacother. 2016;83:271–276. doi: 10.1016/j.biopha.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 182.Zhang L., Hill R.P. Hypoxia enhances metastatic efficiency by up-regulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res. 2004;64:4180–4189. doi: 10.1158/0008-5472.CAN-03-3038. [DOI] [PubMed] [Google Scholar]
- 183.Mairinger F.D., Walter R.F., Ting S., Vollbrecht C., Kollmeier J., Griff S., Hager T., Mairinger T., Christoph D.C., Theegarten D., et al. Mdm2 protein expression is strongly associated with survival in malignant pleural mesothelioma. Future Oncol. 2014;10:995–1005. doi: 10.2217/fon.13.261. [DOI] [PubMed] [Google Scholar]
- 184.Chen Y., Wang D.D., Wu Y.P., Su D., Zhou T.Y., Gai R.H., Fu Y.Y., Zheng L., He Q.J., Zhu H., et al. MDM2 promotes epithelial-mesenchymal transition and metastasis of ovarian cancer SKOV3 cells. Br. J. Cancer. 2017;117:1192–1201. doi: 10.1038/bjc.2017.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Akhoondi S., Sun D., von der L.N., Apostolidou S., Klotz K., Maljukova A., Cepeda D., Fiegl H., Dafou D., Marth C., et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 186.Hagedorn M., Delugin M., Abraldes I., Allain N., Belaud-Rotureau M.A., Turmo M., Prigent C., Loiseau H., Bikfalvi A., Javerzat S. FBXW7/hCDC4 controls glioma cell proliferation in vitro and is a prognostic marker for survival in glioblastoma patients. Cell Div. 2007;2:9. doi: 10.1186/1747-1028-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jin X., Yang C., Fan P., Xiao J., Zhang W., Zhan S., Liu T., Wang D., Wu H. CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J. Biol. Chem. 2017;292:6269–6280. doi: 10.1074/jbc.M116.764407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li Q., Li Y., Li J., Ma Y., Dai W., Mo S., Xu Y., Li X., Cai S. FBW7 suppresses metastasis of colorectal cancer by inhibiting HIF1alpha/CEACAM5 functional axis. Int. J. Biol. Sci. 2018;14:726–735. doi: 10.7150/ijbs.24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Imaizumi T., Kuramoto T., Matsunaga K., Shichijo S., Yutani S., Shigemori M., Oizumi K., Itoh K. Expression of the tumor-rejection antigen SART1 in brain tumors. Int. J. Cancer. 1999;83:760–764. doi: 10.1002/(SICI)1097-0215(19991210)83:6<760::AID-IJC11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 190.Kawamoto M., Shichijo S., Imai Y., Imaizumi T., Koga T., Yanaga H., Itoh K. Expression of the SART-1 tumor rejection antigen in breast cancer. Int. J. Cancer. 1999;80:64–67. doi: 10.1002/(SICI)1097-0215(19990105)80:1<64::AID-IJC13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 191.Sasatomi T., Yamana H., Shichijo S., Tanaka S., Okamura T., Ogata Y., Itoh K., Shirouzu K. Expression of the SART1 tumor-rejection antigens in colorectal cancers. Dis. Colon Rectum. 2000;43:1754–1758. doi: 10.1007/BF02236863. [DOI] [PubMed] [Google Scholar]
- 192.Wu H., Hao A., Cui H., Wu W., Yang H., Hu B., Li P. TRAF6 expression is associated with poorer prognosis and high recurrence in urothelial bladder cancer. Oncol. Lett. 2017;14:2432–2438. doi: 10.3892/ol.2017.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Han F., Zhang L., Qiu W., Yi X. TRAF6 promotes the invasion and metastasis and predicts a poor prognosis in gastric cancer. Pathol. Res. Pract. 2016;212:31–37. doi: 10.1016/j.prp.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 194.Liu X., Wang Z., Zhang G., Zhu Q., Zeng H., Wang T., Gao F., Qi Z., Zhang J., Wang R. High TRAF6 Expression Is Associated with Esophageal Carcinoma Recurrence and Prompts Cancer Cell Invasion. Oncol. Res. 2017;25:485–493. doi: 10.3727/096504016X14749340314441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rong Y., Wang D., Wu W., Jin D., Kuang T., Ni X., Zhang L., Lou W. TRAF6 is over-expressed in pancreatic cancer and promotes the tumorigenicity of pancreatic cancer cells. Med. Oncol. 2014;31:260. doi: 10.1007/s12032-014-0260-9. [DOI] [PubMed] [Google Scholar]
- 196.Luo Z., Zhang X., Zeng W., Su J., Yang K., Lu L., Lim C.B., Tang W., Wu L., Zhao S., et al. TRAF6 regulates melanoma invasion and metastasis through ubiquitination of Basigin. Oncotarget. 2016;7:7179–7192. doi: 10.18632/oncotarget.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fay M.J., Longo K.A., Karathanasis G.A., Shope D.M., Mandernach C.J., Leong J.R., Hicks A., Pherson K., Husain A. Analysis of CUL-5 expression in breast epithelial cells, breast cancer cell lines, normal tissues and tumor tissues. Mol. Cancer. 2003;2:40. doi: 10.1186/1476-4598-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhu Y., Li L., Hou D., Ouyang Y., Guo X., Wang Y., Li J., Gong K. MicroRNA-19a regulates the proliferation, migration and invasion of human gastric cancer cells by targeting CUL5. Arch. Biochem. Biophys. 2019;662:93–100. doi: 10.1016/j.abb.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 199.Devor E.J., Schickling B.M., Reyes H.D., Warrier A., Lindsay B., Goodheart M.J., Santillan D.A., Leslie K.K. Cullin-5, a ubiquitin ligase scaffold protein, is significantly underexpressed in endometrial adenocarcinomas and is a target of miR-182. Oncol. Rep. 2016;35:2461–2465. doi: 10.3892/or.2016.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu X.M., Wang X.B., Chen M.M., Liu T., Li Y.X., Jia W.H., Liu M., Li X., Tang H. MicroRNA-19a and -19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 2012;322:148–158. doi: 10.1016/j.canlet.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 201.Ma C., Qi Y., Shao L., Liu M., Li X., Tang H. Downregulation of miR-7 upregulates Cullin 5 (CUL5) to facilitate G1/S transition in human hepatocellular carcinoma cells. IUBMB Life. 2013;65:1026–1034. doi: 10.1002/iub.1231. [DOI] [PubMed] [Google Scholar]
- 202.Lu Y., Chu A., Turker M.S., Glazer P.M. Hypoxia-induced epigenetic regulation and silencing of the BRCA1 promoter. Mol. Cell Biol. 2011;31:3339–3350. doi: 10.1128/MCB.01121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Paul A., Paul S. The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front. Biosci. 2014;19:605–618. doi: 10.2741/4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hu T., Zhang J., Sha B., Li M., Wang L., Zhang Y., Liu X., Dong Z., Liu Z., Li P., et al. Targeting the overexpressed USP7 inhibits esophageal squamous cell carcinoma cell growth by inducing NOXA-mediated apoptosis. Mol. Carcinog. 2019;58:42–54. doi: 10.1002/mc.22905. [DOI] [PubMed] [Google Scholar]
- 205.Su D., Ma S., Shan L., Wang Y., Wang Y., Cao C., Liu B., Yang C., Wang L., Tian S., et al. Ubiquitin-specific protease 7 sustains DNA damage response and promotes cervical carcinogenesis. J. Clin. Investig. 2018;128:4280–4296. doi: 10.1172/JCI120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang X., Zhang Q., Wang Y., Zhuang H., Chen B. Clinical Significance of Ubiquitin Specific Protease 7 (USP7) in Predicting Prognosis of Hepatocellular Carcinoma and its Functional Mechanisms. Med. Sci. Monit. 2018;24:1742–1750. doi: 10.12659/MSM.909368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu Y., Bedard N., Chevalier S., Wing S.S. Identification of distinctive patterns of USP19-mediated growth regulation in normal and malignant cells. PLoS ONE. 2011;6:e15936. doi: 10.1371/journal.pone.0015936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu M., Tu H.Q., Chang Y., Tan B., Wang G., Zhou J., Wang L., Mu R., Zhang W.N. USP19 deubiquitinates HDAC1/2 to regulate DNA damage repair and control chromosomal stability. Oncotarget. 2017;8:2197–2208. doi: 10.18632/oncotarget.11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang S., Liu L., Cao C., Song N., Wang Y., Ma S., Zhang Q., Yu N., Ding X., Yang F., et al. USP52 acts as a deubiquitinase and promotes histone chaperone ASF1A stabilization. Nat. Commun. 2018;9:1285. doi: 10.1038/s41467-018-03588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang J.H., Wei W., Guo Z.X., Shi M., Guo R.P. Decreased Cezanne expression is associated with the progression and poor prognosis in hepatocellular carcinoma. J. Transl. Med. 2015;13:41. doi: 10.1186/s12967-015-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pang Z., Cui L., Ding N., Zhu L., Qu X., Dong W., Du J., Liu Q. Expressions of insulin-like growth factor receptor-1 and cezanne-1 in lung adenocarcinoma. Med. Oncol. 2017;34:78. doi: 10.1007/s12032-017-0934-1. [DOI] [PubMed] [Google Scholar]
- 212.Zhang B., Wang H., Yang L., Zhang Y., Wang P., Huang G., Zheng J., Ren H., Qin S. OTUD7B and NIK expression in non-small cell lung cancer: Association with clinicopathological features and prognostic implications. Pathol. Res. Pract. 2016;212:893–898. doi: 10.1016/j.prp.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 213.Wang B., Jie Z., Joo D., Ordureau A., Liu P., Gan W., Guo J., Zhang J., North B.J., Dai X., et al. TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling. Nature. 2017;545:365–369. doi: 10.1038/nature22344. [DOI] [PMC free article] [PubMed] [Google Scholar]