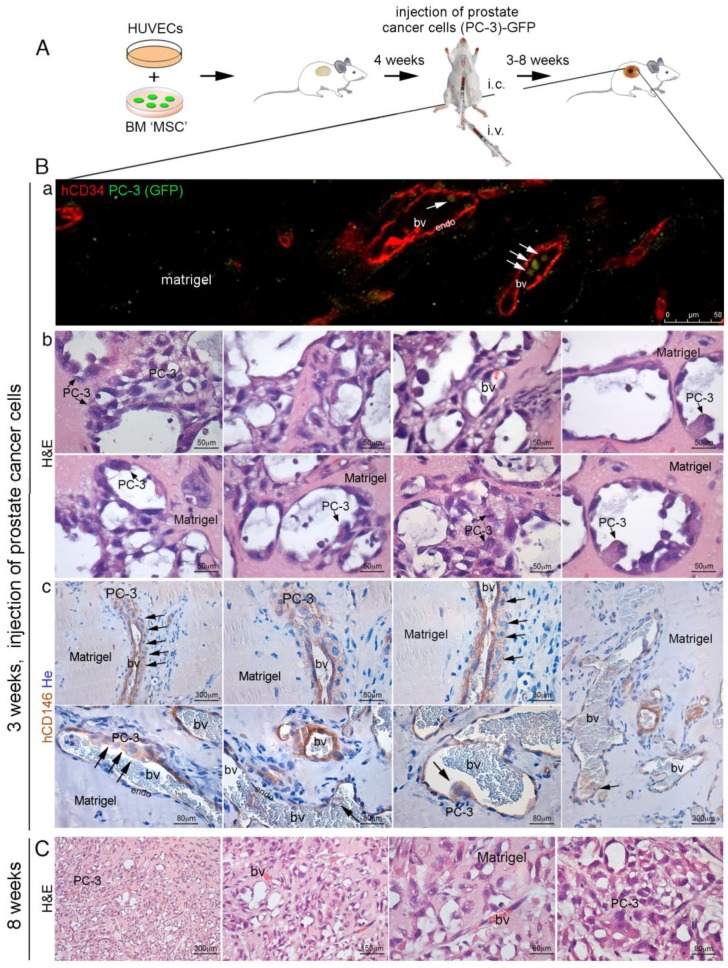
Figure 8.
(A) in vivo heterotopic co-transplants of hBMSCs in MatrigelTM with HUVECs and injection of PC-3 cells; (B) implants generated by hBMSCs and HUVECs in MatrigelTM efficiently formed functional blood vessels in 3–4 weeks. At this time, PC-3 cells were blood-injected in SCID/beige mice. Cancer cells colonized the implants generating metastasis. (a) representative images of implants (hBMSCs/HUVECs in MatrigelTM) harvested at 3–4 weeks after injection of PC-3 cells; (b) co-transplants of hBMSCs in Matrigel with HUVECs generated an extensive functional network of capillary-like blood vessels (bv), as indicated by blood host perfusion, in the presence of red blood cells (RBCs). These vessels were composed by two cell layers: an inner continuous hCD34+ endothelial layer (endo), coated by an outer subluminal, thin, discontinuous layer of hCD146+ mural cells. In many instances, the blood vessels were coated with a multilayer of hCD146+ PC-3 cells, wrapped around the outer surface of vessels or near vessels in association with the layer of hCD146+ mural cells as demonstrated by hCD146 immunostaining and hematoxylin counterstaining. Early metastatic PC-3 cell clusters were regularly associated with blood vessels. (c) By fluorescence microscopy, single GFP-labeled PC-3 cells could be observed inside blood vessels; (C) co-transplants of hBMSCs in MatrigelTM along with HUVECs resulted in the formation of extensive metastases after eight weeks from i.c. injected PC3 cells, as demonstrated by H&E staining. H&E, Hematoxylin/Eosin; bv, blood vessels; RBCs, red blood cells; endo, endothelial cells. He, Hematoxylin counterstaining. Scale bar = 50, 80, 150, 300 μm.