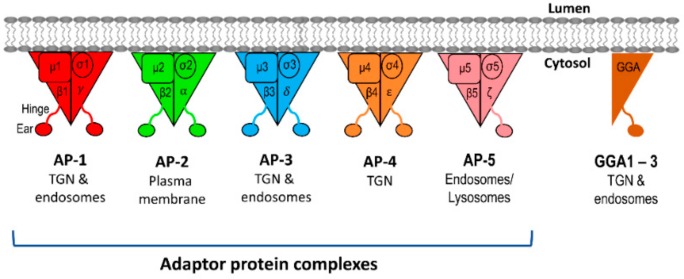
Figure 4.
Cargo adaptor proteins: Five adaptor protein (AP) complexes, AP-1 (red), AP-2 (green), AP-3 (blue), AP-4 (orange), and AP-5 (pink), have been identified to date in higher eukaryotes. Each heterotetrameric AP complex comprises two ~100 kDa large subunits (β1–5, and either α, γ, δ, ε, or ζ), one ~50 kDa medium subunit (μ1–5), and one ~20 kDa small subunit (σ1–5). Together, they form the AP core, which is important for membrane recruitment and cargo sorting motif recognition. The C-termini of both large subunits of each AP complex give rise to the hinge and ear domains for further recruitment of accessory proteins. AP-1 is localized at the TGN/recycling endosomes and regulates bidirectional transport. AP-1 also regulates basolateral sorting in polarized cells. AP-2 is responsible for endocytosis of cargoes from the cell surface. AP-3 is localized at the TGN/early endosomes and regulates transport to the late endosomes/lysosomes. AP-4 is localized at the TGN and regulates cargo trafficking from the TGN to the early endosomes. AP-5 is localized at the late endosomes/lysosomes, and the trafficking pathway it regulates is still unclear. The three Golgi-localized γ-ear containing Arf binding isoforms, GGA1, GGA2, and GGA3 (brown), are monomeric and have similar structural protein folding to the ear domain of AP-1γ subunit. GGAs are localized at the TGN and endosomes.