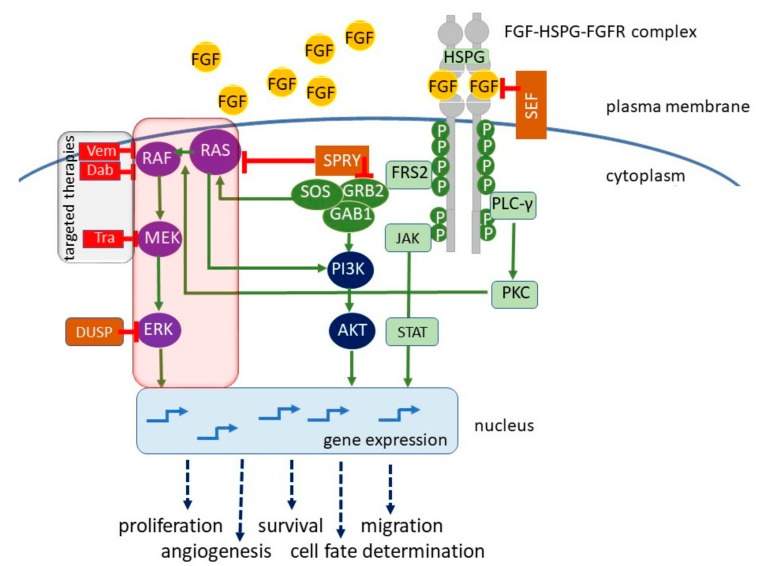
Figure 1.
Fibroblast growth factor receptors (FGFRs) are highly conserved transmembrane receptors consisting of three extracellular immunoglobulin-like (Ig-like) domains, a transmembrane helical region, and a cytoplasmic region with kinase activity. The fibroblast growth factor (FGF) ligand and its cofactor heparin sulfate proteoglycan (HSPG) bind to FGFR monomers, leading to dimerization and tyrosine cross-autophosphorylation of the cytoplasmic domain. This induces various signaling pathways, resulting in cellular proliferation, survival, migration, angiogenesis, and cell fate determination in embryogenesis and in response to microenvironmental signals, including therapeutics. FGF/FGFR signaling can be stimulated in a paracrine manner, mainly in physiological settings, or in an autocrine manner as demonstrated in various cancers. In melanoma, FGF/FGFR signaling is largely suppressed by mutation-driven enhanced activity of the RAS (Rat sarcoma oncogene)/BRAF (v-Raf murine sarcoma viral oncogene homolog B)/MEK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) pathway (red framed). Melanoma cells that acquire the ability to secrete FGFs and stimulate FGFR in a paracrine or autocrine manner can contribute to angiogenesis and cell-fate decisions involving transitions between different phenotypes, including phenotypes resistant to targeted therapies (grey framed). Dab, dabrafenib; DUSP, dual-specificity phosphatase; FRS2, FGFR substrate 2; GAB1, GRB2-associated binding protein 1; GRB2, growth factor receptor protein 2; JAK, Janus kinase; PKC, protein kinase C; PLC-γ, phospholipase C gamma; SOS, son of sevenless; SEF, similar expression to FGF; SPRY, Sprouty; STAT, signal transducer and activator of transcription; Tra, trametinib; Vem, vemurafenib.