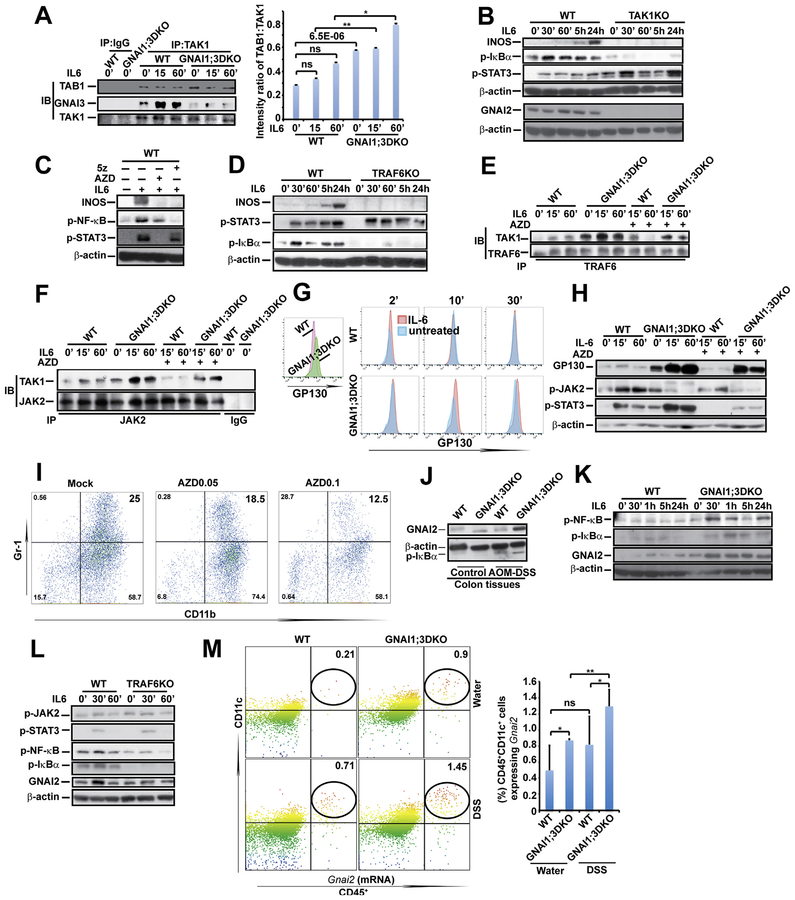
Figure 4.
GNAI1;3 deficiency leads to enhanced TAK1–TAB1 interaction and IL6-induced JAK activity-dependent interactions among TAK1, JAK2, and TRAF6, which are required for IL6 activation of NF-ĸB, and GNAI2, GP130, and INOS up-regulation. (A) IP/IB and quantified analysis for interactions of TAK1 with TAB1 and GNAI3 (n = 3). (B) IB analysis for INOS, p-IĸBα(S32), p-STAT3(Y705), GNAI2, and β-actin in WT and TAK1KO MEFs. (C) IB analysis for INOS, p-NF-ĸBp65(S536), p-STAT3(Y705), and β-actin in WT MEFs treated with AZD or 5z. (D) IB analysis for INOS, p-IĸBα(S32), p-STAT3(Y705), and b-actin in WT and TRAF6KO MEFs, (E) IP/IB analysis for TAK1–TRAF6 interaction in WT and GNAI1;3DKO MEFs. (F) IP/IB analysis for TAK1–JAK2 interaction in WT and GNAI1;3DKO MEFs. (G) Representative FACS plots of GP130 on the surface of IL6-treated WT and GNAI1;3DKO MEFs. (H) IB for GP130, p-JAK2(Y1007/8), p-STAT3(Y705), and β-actin in treated WT and GNAI1;3DKO MEFs. (I) Representative FACS plots of CD11b+Gr-1+ WT BMDSCs differentiated with GM-CSF and IL6 in the presence or absence of AZD. (J) IB for GNAI2, p-IĸBα(S32), and β-actin in indicated WT and GNAI1;3DKO colonic tissues. (K) IB for GNAI2, p-IĸBα(S32), p-NF-ĸBp65(S536), and β-actin in WT and GNAI1;3DKO MEFs. (L) IB for GNAI2, p-IĸBa(S32), p-JAK2(Y1007/8), p-NF-ĸBp65(S536), p-STAT3(Y705), and β-actin in WT and TRAF6KO MEFs. (M) PrimeFlow analysis of Gnai2 mRNA expression in CD45+CD11c+ colonic LP isolated from indicated WT and GNAI1;3DKO mice on day 10 (n = 6). Data are mean ± standard deviation. *P < .05, **P < .01, ***P < .001; 2-tailed Student t test. All data in A–L are representative of 2 or 3 independent experiments. 5z, 5z-7-oxozeaenol; FACS, fluorescence-activated cell sorting; ns, not significant; p-, phosphorylated.