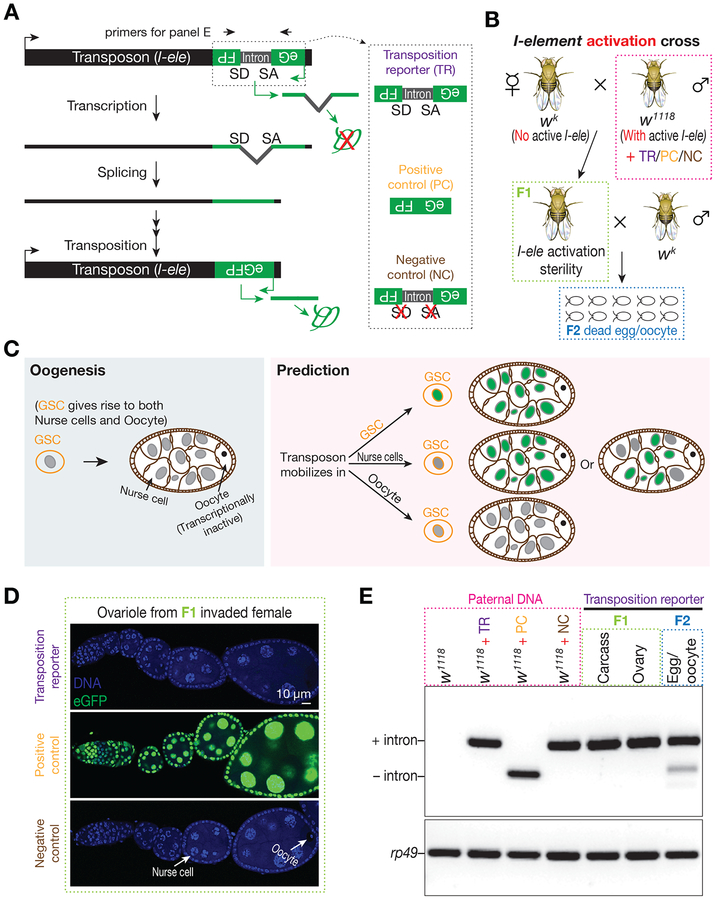
Figure 1. Tracking I-element mobilization during oogenesis with an eGFP reporter reveals oocytes are preferentially targeted for transposition.
(A) Schematic desgin of eGFP reporter to track I-element mobilization. An eGFP reporter is inserted into the 3’ UTR of I-element sequences in the antisense direction. eGFP expression is blocked by an intron, which is in the same orientation of transposon and therefore is unable to be spliced during eGFP transcription. Only transposition can generate a copy of DNA without the dirupting intron, thereby producing eGFP signal. A construct without disrupting intron serves as positive control to test the potential sensitivity of reporter. A construct with mutations on the splicing acceptor and donor sites serves as negative control.
(B) The genetic setup to trigger I-element mobilization. Without protection from the I-element-silencing piRNAs, which are typically maternally deposited, paternally inherited I-elements become active in invaded progeny. These offspring were crossed with wk males to purify the oocytes from their ovaries. The I-element containing fathers carry either transposition reporter or one of the control constructs.
(C) A simplified cartoon to illustrate Drosophila oogenesis and the potential outcomes from reporter.
(D) Detecting eGFP expression in invaded ovaries carrying each construct. Transposition reporter does not give signal from any ovarian cells in I-element activated progeny. The strong signal from positive control construct highlights the potentially high sensitivity of reporter.
(E) Probing transposition events by PCR. Transpositions in oocyte generate intron-removed DNA, which gives rise to a short PCR product. Primers are depicted in panel A. Samples were boxed with different color to match the corresponding animals depicted in panel B.